8.51006
HBTU
≥99.0% (HPLC), for peptide synthesis, Novabiochem®
Sinonimo/i:
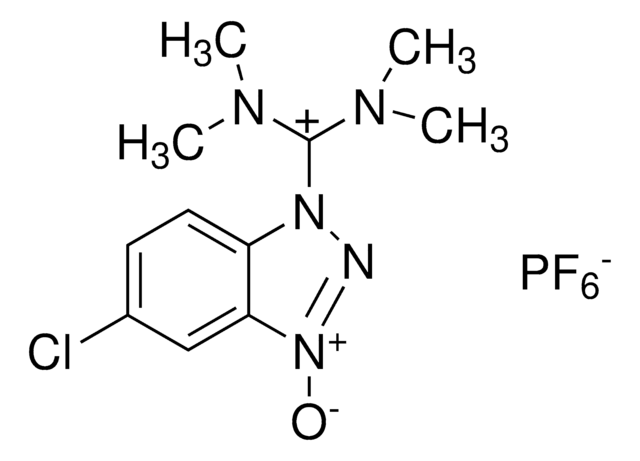
HBTU, 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate
About This Item
Prodotti consigliati
product name
HBTU, 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate Novabiochem®
Livello qualitativo
Nome Commerciale
Novabiochem®
Saggio
≥99.0% (HPLC)
Forma fisica
powder
Potenza
>2000 mg/kg LD50, oral (Rat)
Impiego in reazioni chimiche
reaction type: Coupling Reactions
Produttore/marchio commerciale
Novabiochem®
pH
4.1 (1.6 g/L in H2O)
Punto di fusione
250 °C
Solubilità
1.6 g/L
applicazioni
peptide synthesis
Temperatura di conservazione
2-8°C
InChI
1S/C11H16N5O/c1-14(2)11(15(3)4)17-16-10-8-6-5-7-9(10)12-13-16/h5-8H,1-4H3/q+1
CLZISMQKJZCZDN-UHFFFAOYSA-N
Descrizione generale
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents
Literature references
[1] R. Knorr, et al. (1989) Tetrahedron Lett., 30, 1927.
[2] M. S. Bernatowicz, et al. (1989) Tetrahedron Lett., 30, 4645.
[3] D. Ambrosius, et al. (1989) Biol. Chem. Hoppe-Seyler, 370, 217.
[4] C. G. Fields, et al. (1991) Pept. Res., 4, 95.
[5] A. G. Beck-Sickinger, et al. (1991) Pept. Res., 4, 88.
[6] G. E. Reid, et al. (1992) Anal. Biochem., 200, 301.
[7] G. B. Fields, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 1st International Symposium′, R. Epton (Eds), SPCC UK Ltd., Birmingham, 1990, pp. 241.
[8] P. A. Baybayan, et al. in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 566.
[9] J. J. Dudash, et al. (1993) Synth. Commun., 23, 349.
Applicazioni
- Synthesis of Quinoxaline Derivatives Using HBTU: A study highlighting the use of HBTU as a Lewis acid catalyst for synthesizing quinoxaline derivatives, presenting a mild and green protocol (Popatkar and Meshram, 2020).
- Efficient Conversion of Carboxylic Acids into Benzimidazoles: Details an HBTU-promoted methodology for converting carboxylic acids into benzimidazoles in a one-pot strategy (Barasa and Yoganathan, 2018).
- Synthesis of Malonyl-linked Glycoconjugates: Discusses the use of HBTU in the synthesis of glycoconjugates, comparing its efficiency with other reagents (Nörrlinger et al., 2016).
Linkage
Risultati analitici
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Note legali
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Skin Sens. 1A
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.



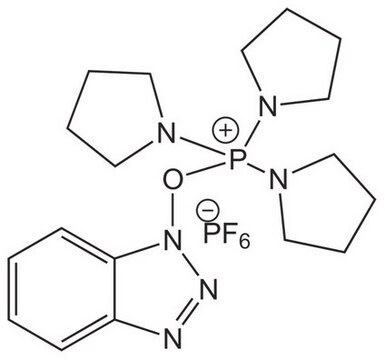
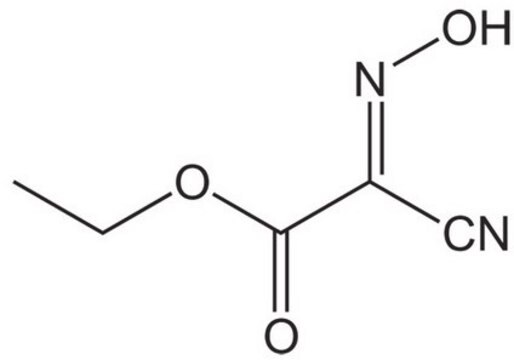
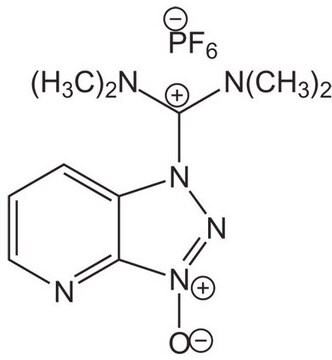
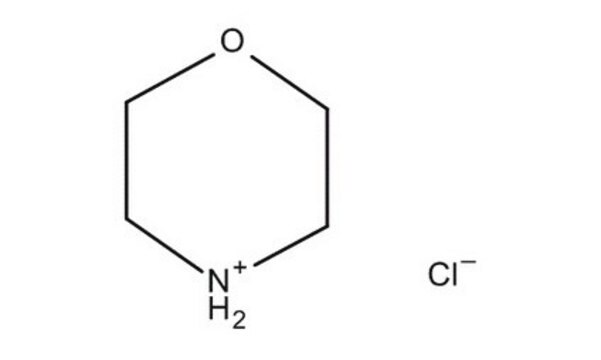

![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)