5.09584
ISR Inhibitor, ISRIB
Sinonimo/i:
ISR Inhibitor, ISRIB, trans-2-(4-Chlorophenoxy)-N-(4-(2-(4-chlorophenoxy)acetylamino)cyclohexyl)acetamide, Integrated Stress Response Inhibitor, UPR Inhibitor, eIF2α (pS51) Signaling Inhibitor, eIF2S1 (pS51) Signaling Inhibitor, eIF2α (pS51) Signaling Inhibitor, eIF2S1 (pS51) Signaling Inhibitor, trans-2-(4-Chlorophenoxy)-N-(4-(2-(4-chlorophenoxy)acetylamino)cyclohexyl)acetamide, Integrated Stress Response Inhibitor, UPR Inhibitor
About This Item
Prodotti consigliati
Saggio
≥93% (HPLC)
Livello qualitativo
Forma fisica
powder
Produttore/marchio commerciale
Calbiochem®
Condizioni di stoccaggio
OK to freeze
protect from light
Colore
light beige
Solubilità
DMSO: 10 mg/mL
Temperatura di conservazione
2-8°C
Stringa SMILE
C1CC(CCC1NC(=O)COC2=CC=C(C=C2)Cl)NC(=O)COC3=CC=C(C=C3)Cl
Descrizione generale
Azioni biochim/fisiol
Confezionamento
Attenzione
Ricostituzione
Altre note
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.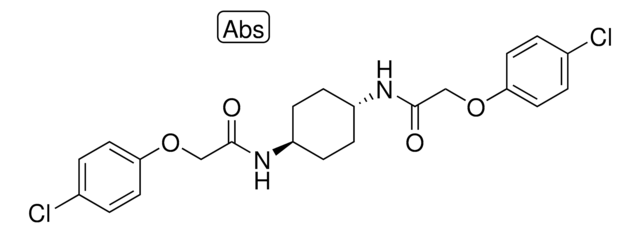

![PERK Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)