1.03164
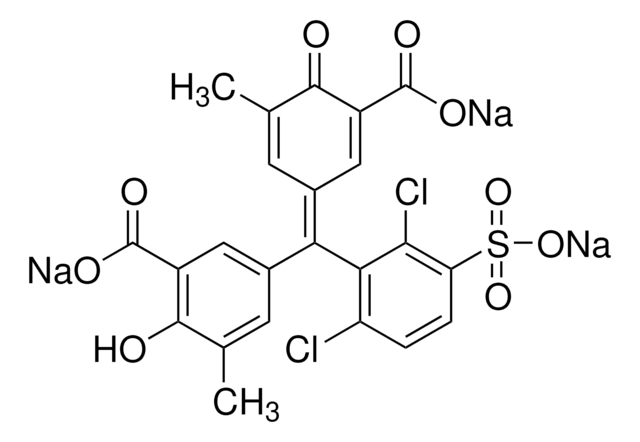
Eriochrome cyanine R (C.I. 43820)
for analysis (reagent for aluminium)
Sinonimo/i:
Eriochrome cyanine R (C.I. 43820)
About This Item
Prodotti consigliati
Livello qualitativo
Potenza
>2000 mg/kg LD50, oral (Rat)
Perdita
≤10% loss on drying, 110°C
pH
9.1 (24 °C, 10 g/L in H2O)
Solubilità
420 g/L
Densità bulk
590 kg/m3
λmax
434-440 nm (buffer pH 7.0)
Temperatura di conservazione
2-30°C
InChI
1S/C23H18O9S.Na/c1-11-7-13(9-15(19(11)24)21(26)27)23(14-8-12(2)20(25)16(10-14)22(28)29)17-5-3-4-6-18(17)33(30,31)32-23;/h3-10,24-25H,1-2H3,(H,26,27)(H,28,29);/q;+1
HJEGHSWGWSAZBS-UHFFFAOYSA-N
Categorie correlate
Applicazioni
- Determination of Food Oxalates Using Silica-Titania Xerogel Modified with Eriochrome Cyanine R.: This research explores a novel method to determine oxalate concentrations in food using Eriochrome Cyanine R, highlighting the dye′s potential in enhancing analytical sensitivity and specificity (Morosanova et al., 2018).
- Development of a Dispersive Liquid-Liquid Microextraction Method Combined with UV-Visible Spectrophotometry for Determination of Trace Aluminum(III) in Water, Wastewater, Food, Biological, and Pharmaceutical Samples.: This study demonstrates a new method utilizing Eriochrome Cyanine R for tracing aluminum levels across various samples, showcasing its utility in environmental and health-related chemical analyses (Birgani et al., 2017).
- Direct, sensitive determination of trace amounts of dissolved ferric iron in natural water by light absorption ratio variation spectrometry.: Though not directly mentioning Eriochrome Cyanine R, this article focuses on related spectrometric techniques that can be adapted for dyes like Eriochrome Cyanine R to detect iron in natural water sources (Gao et al., 2005).
- Determination of vanadium by solid-phase spectrophotometry after its preconcentration as an Eriochrome Cyanine R complex on a dextran-type exchanger.: This research introduces a method for vanadium determination using Eriochrome Cyanine R, underlining the dye′s role in the preconcentration and quantification of vanadium in complex matrices (Boudra et al., 1995).
Risultati analitici
Absorption maximum λmax. (buffer pH 7.0): 434 - 440 nm
Spec. Absorptivity A 1%/1cm (λmax; 0.02 g/l; buffer pH 7.0; calc. on dried substance): 130 - 230
Loss on drying (110 °C): ≤ 10 %
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.