1.01115
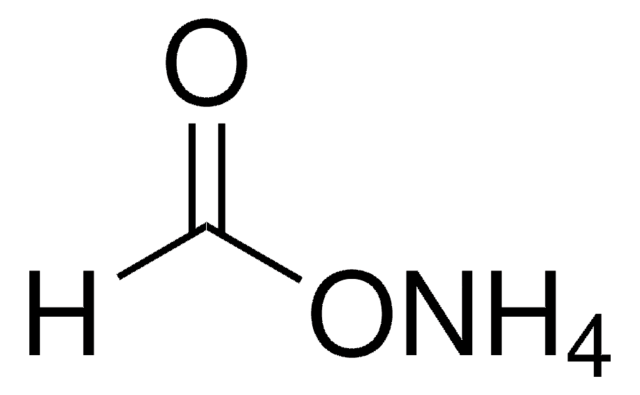
Ammonium acetate
EMPLURA®
Sinonimo/i:
Ammonium acetate
About This Item
Prodotti consigliati
Livello qualitativo
Nome Commerciale
EMPLURA®
Saggio
≥96.0% (alkalimetric)
Forma fisica
solid
Residuo alla calcinazione
≤0.02% (as sulfate)
pH
6.7-7.3 (25 °C, 50 g/L in H2O)
Punto di fusione
114 °C
Solubilità
1480 g/L
Densità
1.17 g/cm3 at 20 °C
Densità bulk
410 kg/m3
Anioni in tracce
chloride (Cl-): ≤0.002%
sulfate (SO42-): ≤0.01%
Cationi in tracce
Fe: ≤0.001%
heavy metals (as Pb): ≤0.0005%
Temperatura di conservazione
15-25°C
InChI
1S/C2H4O2.H3N/c1-2(3)4;/h1H3,(H,3,4);1H3
USFZMSVCRYTOJT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- One-Pot, Four-Component Reaction for the Design, Synthesis, and SAR Studies of Novel Pyridines for Insecticidal Bioefficacy Screening against Cowpea Aphid (Aphis craccivora): This study explores the application of ammonium acetate in facilitating the synthesis of novel pyridine derivatives, evaluated for their insecticidal properties against Aphis craccivora (Alkorbi et al., 2024).
- A green analytical method for the simultaneous determination of 17 perfluoroalkyl substances (PFAS) in human serum and semen by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS): This research demonstrates the use of ammonium acetate in UPLC-MS/MS for detecting PFAS in biological samples, highlighting its role in environmental and health-related analytical chemistry (Di Giorgi et al., 2024).
- Innovation of 6-sulfonamide-2H-chromene derivatives as antidiabetic agents targeting α-amylase, α-glycosidase, and PPAR-γ inhibitors with in silico molecular docking simulation: Ammonium acetate was utilized in the synthesis process of new chromene derivatives, showing potential as antidiabetic agents by inhibiting α-amylase and α-glycosidase activities (Thabet et al., 2024).
- Regulation of Tetramethylpyrazine Formation by the Phenolics-Fenton Coupled Redox Cycling System: This article presents a study on the role of ammonium acetate in the formation of tetramethylpyrazine via redox cycling, an important reaction in food chemistry and possibly in synthesizing complex organic molecules (Xu et al., 2024).
- Simultaneous determination of unecritinib (TQ-B3101) and its active metabolite crizotinib in rat plasma by LC-MS/MS: An application to pharmacokinetic studies: This paper details the use of ammonium acetate in enhancing LC-MS/MS methodologies for pharmacokinetic studies, exemplifying its critical role in the analysis of pharmaceutical compounds (Wang et al., 2024).
Risultati analitici
Identity: passes test
pH-value (5 %; water): 6.0 - 7.5
Chloride (Cl): ≤ 0.002 %
Sulfate (SO₄): ≤ 0.01 %
Heavy metals (as Pb): ≤ 0.0005 %
Fe (Iron): ≤ 0.001 %
Residue on ignition (as sulfate): ≤ 0.02 %
Water: ≤ 2.5 %
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.