800725P
Avanti
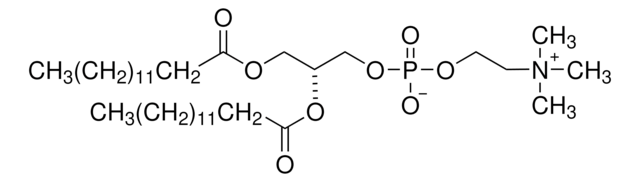
N-P Tyrosine PA
Avanti Research™ - A Croda Brand 800725P, powder
Sinonimo/i:
N-palmitoyl-tyrosine phosphoric acid (ammonium salt)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C25H48N3O7P
Numero CAS:
Peso molecolare:
533.64
Numero MDL:
Codice UNSPSC:
12352211
NACRES:
NA.25
Prodotti consigliati
Saggio
>99% (TLC)
Stato
powder
Confezionamento
pkg of 1 × 1 mg (800725P-1mg)
Produttore/marchio commerciale
Avanti Research™ - A Croda Brand 800725P
Tipo di lipide
phospholipids
cardiolipins
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
Descrizione generale
Lysophosphatidic acid (LPA) receptor modulators include N-palmitoyl serine phosphoric acid and N-palmitoyl-tyrosine phosphoric acid. N-palmitoyl serine phosphoric acid and N-palmitoyl-tyrosine phosphoric acid are competitive inhibitors of the LPA receptor in Xenopus oocytes. However, in mammalian cells, N-palmitoyl-tyrosine phosphoric acid may act as an agonist for the LPA receptor. LPA is a lipid mediator that acts similar to growth factors through G-protein coupled plasma membrane receptors. LPA may play a role in platelet aggregation, smooth muscle contraction, vasoactive changes, cytoskeletal reorganization and cell proliferation.
Confezionamento
5 mL Amber Glass Screw Cap Vial (800725P-1mg)
Nota sulla preparazione
Product use: N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acid can be used for cell studies. dissolved these lipids in 0.1 mL PBS containing 0.1 mg/mL human serum albumin before adding to cells. In X. laevis studies, these LPA inhibitors were dissolved in DMSO at 1 mM and filtered through a 0.45 mM membrane filter before injection.
Note legali
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Codice della classe di stoccaggio
11 - Combustible Solids
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction.
Neidlinger, N.A, et. al.
The Journal of Biological Chemistry, 281, 775-781 (2006)
Inhibitors of lipid phosphatidate receptors: N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acids
Bittman, R, et. al.
Journal of Lipid Research, 37, 391-398 (1996)
Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization
An, S
Molecular Pharmacology, 54, 881-888 (1998)
Inhibitors of lipid phosphatidate receptors: N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acids
Bittman, R
Journal of Lipid Research, 37, 391-398 (1996)
R Bittman et al.
Journal of lipid research, 37(2), 391-398 (1996-02-01)
An improved synthesis of two lipid phosphoric acids, N-palmitoyl-L-serine phosphoric acid (NP-Ser-PA) and N-palmitoyl-L-tyrosine phosphoric acid (NP-Tyr-PA), from the benzyl esters of L-serine and L-tyrosine is described. The sequence of N-acylation, followed by phosphitylation with N, N-diisopropyl dibenzyl phosphoramidite, oxidation
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.