W323713
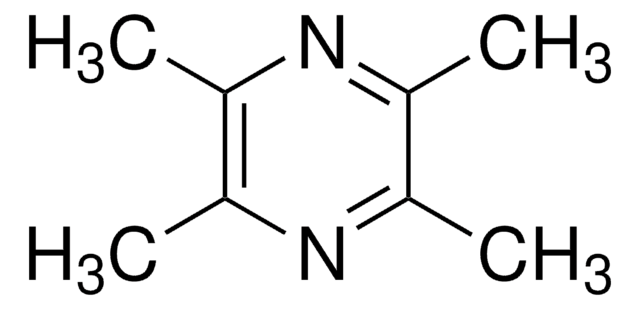
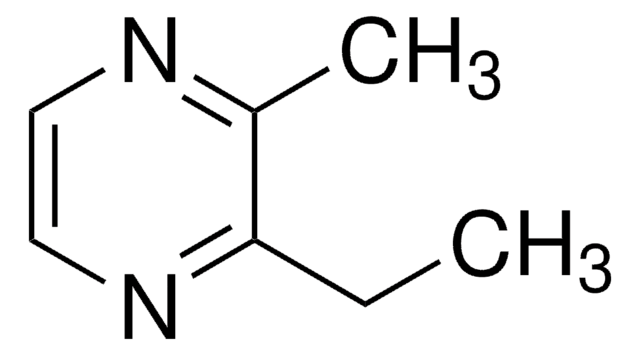
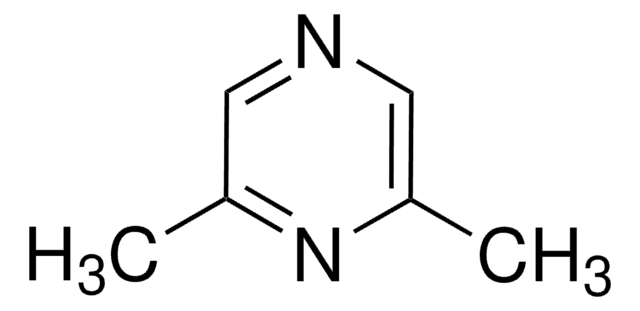
2,3,5,6-Tetramethylpyrazine
natural, ≥98%, FG
Sinonimo/i:
Chuanxingzine, Ligustrazine, Tetrapyrazine
About This Item
Prodotti consigliati
Grado
FG
Fragrance grade
Halal
Kosher
natural
agenzia
follows IFRA guidelines
meets purity specifications of JECFA
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
Saggio
≥98%
Caratteristiche più verdi
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezze
≤2.0% water (Karl Fischer)
P. ebollizione
190 °C (lit.)
Punto di fusione
77-80 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Categoria alternativa più verde
, Aligned
Organolettico
chocolate; coffee; fatty; musty; nutty
Stringa SMILE
Cc1nc(C)c(C)nc1C
InChI
1S/C8H12N2/c1-5-6(2)10-8(4)7(3)9-5/h1-4H3
FINHMKGKINIASC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Booklice Liposcelis bostrychophila are efficiently attracted by the combination of 2,3,5,6-tetramethylpyrazine and ultraviolet light.: This study demonstrates that the combination of 2,3,5,6-tetramethylpyrazine and ultraviolet light effectively attracts booklice, suggesting a potential application for pest management in stored product environments (Tanaka et al., 2024).
- 2,3,5,6-Tetramethylpyrazine protects retinal photoreceptors against endoplasmic reticulum stress by modulating ATF4-mediated inhibition of PRP aggregation.: The research highlights the neuroprotective effects of 2,3,5,6-tetramethylpyrazine, showing its potential in treating retinal diseases by protecting photoreceptors from stress-induced damage (Huang et al., 2021).
- Tetramethylpyrazine-Inducible Promoter Region from Rhodococcus jostii TMP1.: The study identifies a promoter region in Rhodococcus jostii TMP1 that is inducible by tetramethylpyrazine, which could be utilized in genetic engineering and biotechnology applications (Stanislauskienė et al., 2018).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W323713-100G | |
| W323713-SAMPLE-K | 4061834356172 |
| W323713-100G-K | 4061834405672 |
| W323713-1KG | |
| W323713-1KG-K | 4061837528392 |
| W323713-25G | |
| W323713-25G-K | |
| W323713-SAMPLE |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.