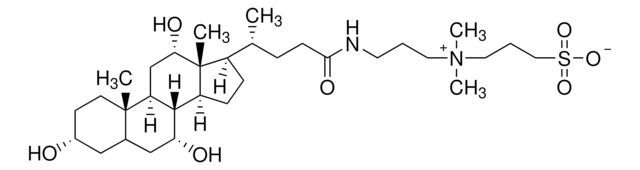
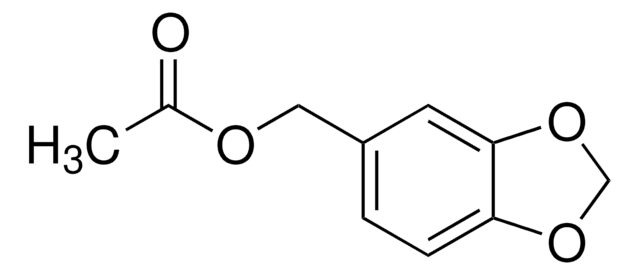
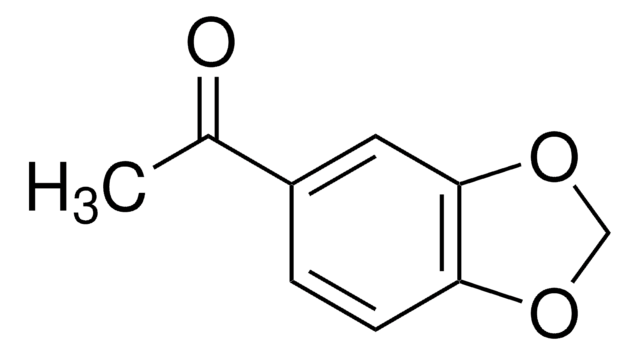
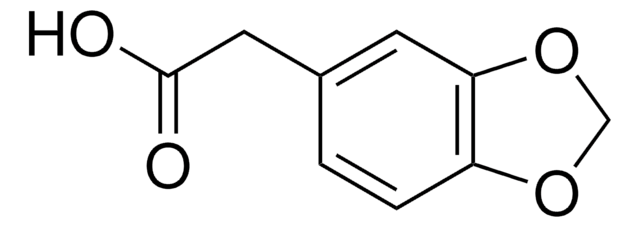
P49805
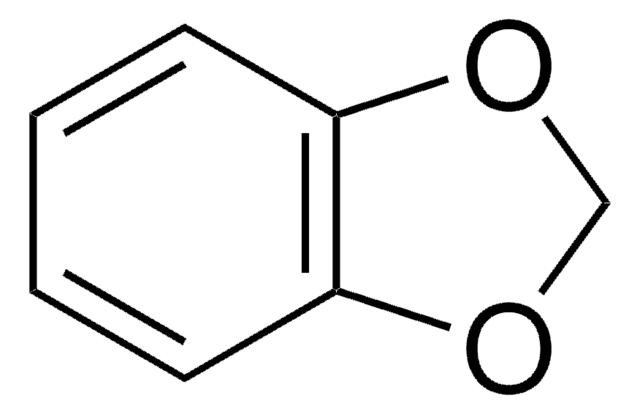
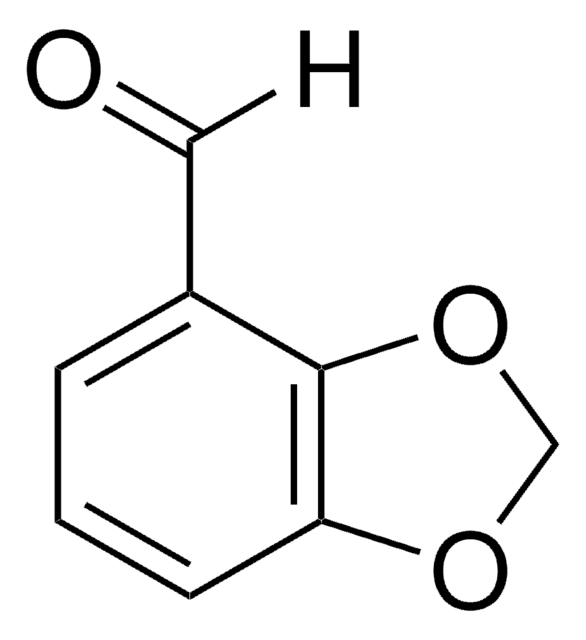
Piperonylic acid
99%
Sinonimo/i:
1,3-Benzodioxole-5-carboxylic acid, 3,4-(Methylenedioxy)benzoic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H6O4
Numero CAS:
Peso molecolare:
166.13
Beilstein:
150206
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Forma fisica
powder
Punto di fusione
229-231 °C (lit.)
Stringa SMILE
OC(=O)c1ccc2OCOc2c1
InChI
1S/C8H6O4/c9-8(10)5-1-2-6-7(3-5)12-4-11-6/h1-3H,4H2,(H,9,10)
VDVJGIYXDVPQLP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
R Ranga Rao et al.
Bioorganic & medicinal chemistry, 17(14), 5170-5175 (2009-06-12)
A bioassay-guided fractionation and chemical examination of antihyperglycemic root extract of Derris indica resulted in isolation and characterization of two new furanoflavanoids (1, 2) along with thirteen known compounds (3-15). Their structures were determined on the basis of extensive spectroscopic
Debabrata Sircar et al.
Journal of plant physiology, 166(13), 1370-1380 (2009-04-04)
Biosynthesis of hydroxybenzoates even at enzymatic level is poorly understood. In this report, effect of feeding of putative biosynthetic precursors and pathway-specific enzyme inhibitors of early phenylpropanoid pathway on p-hydroxybenzoic acid accumulation in chitosan-elicited hairy roots of Daucus carota was
Eyal Shimoni et al.
Journal of biotechnology, 105(1-2), 61-70 (2003-09-27)
Propenylbenzenes are often used as starting materials in the chemical synthesis of aroma compounds and fine chemicals. In the present study, we demonstrate the ability of an Arthrobacter sp. to transform various structures of propenylbenzenes derived from essential oils to
Gisele Adriana Bubna et al.
Journal of plant physiology, 168(14), 1627-1633 (2011-04-15)
The allelopathic effect of caffeic acid was tested on root growth, phenylalanine ammonia-lyase (PAL) and peroxidase (POD) activities, hydrogen peroxide (H(2)O(2)) accumulation, lignin content and monomeric composition of soybean (Glycine max) roots. We found that exogenously applied caffeic acid inhibited
J Chong et al.
Plant physiology, 125(1), 318-328 (2001-01-12)
Salicylic acid (SA) is a key endogenous component of local and systemic disease resistance in plants. In this study, we investigated the role of benzoic acid (BA) as precursor of SA biosynthesis in tobacco (Nicotiana tabacum cv Samsun NN) plants
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.