H43407
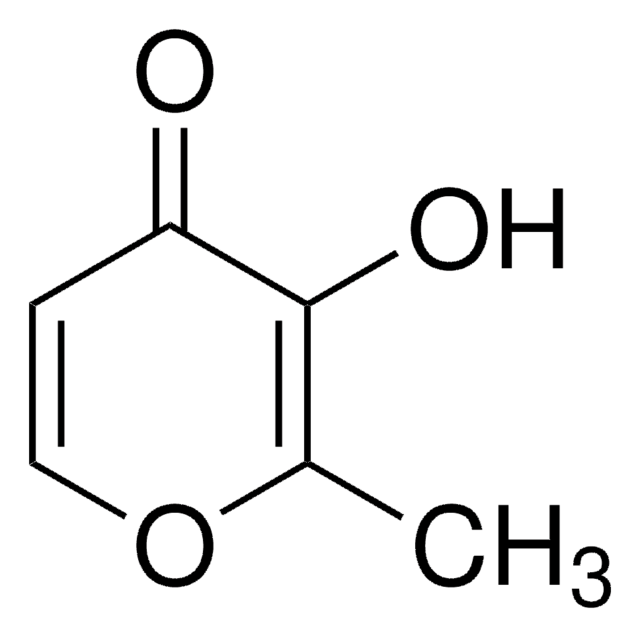
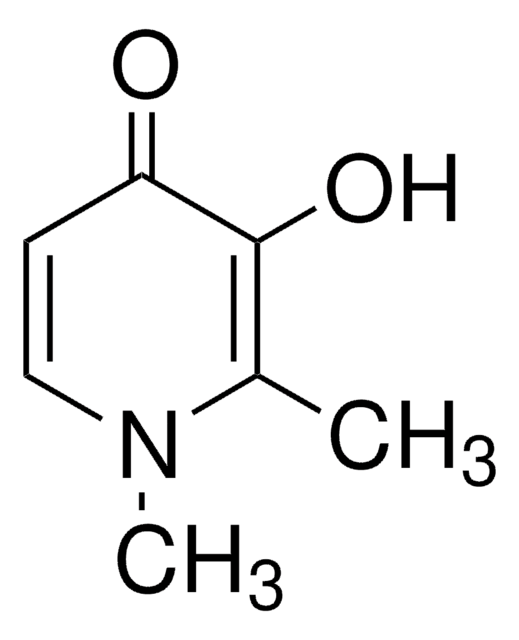
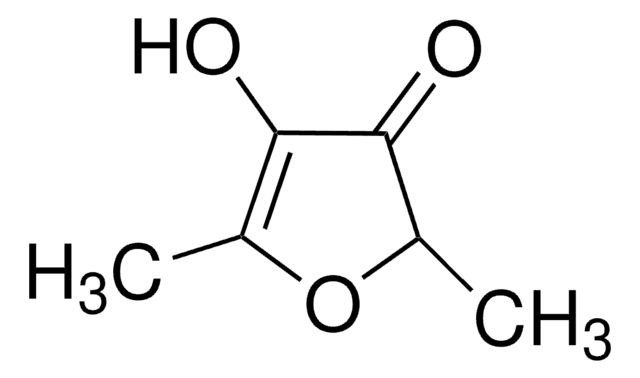
3-Hydroxy-2-methyl-4-pyrone
99%
Sinonimo/i:
3-Hydroxy-2-methyl-4H-pyran-4-one, Larixinic acid, Maltol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H6O3
Numero CAS:
Peso molecolare:
126.11
Beilstein:
112169
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Temp. autoaccensione
1364 °F
Limite di esplosione
25 %
Punto di fusione
160-164 °C (lit.)
Stringa SMILE
CC1=C(O)C(=O)C=CO1
InChI
1S/C6H6O3/c1-4-6(8)5(7)2-3-9-4/h2-3,8H,1H3
XPCTZQVDEJYUGT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Anwar Anwar-Mohamed et al.
Toxicology in vitro : an international journal published in association with BIBRA, 21(4), 685-690 (2007-02-24)
Maltol is used extensively as a flavor-enhancing agent, food preservative, antioxidant, and also in cosmetic and pharmaceutical formulations. However, a number of studies have shown that maltol may induce carcinogenicity and toxicity but the mechanisms involved remain unknown. Therefore, we
Tamás Jakusch et al.
Dalton transactions (Cambridge, England : 2003), (13)(13), 2428-2437 (2009-03-18)
The interactions of various insulin mimetic oxovanadium(IV) compounds with serum proteins were studied in model systems and in ex vivo samples. For the modeling study, an earlier in situ method was extended and applied to the formation of ternary complexes
Sílvia Chaves et al.
Journal of inorganic biochemistry, 114, 38-46 (2012-06-13)
The O,S-donor analogues of maltol and deferiprone (DMHP), respectively, thiomaltol and DMHTP, have been investigated in solution for their iron-complexation ability, as well as their electrochemical behaviors, in the presence and absence of iron, aimed at the rationalization of their
Christoph J Jocher et al.
Inorganic chemistry, 47(18), 7951-7953 (2008-08-19)
The synthesis, characterization, and photophysical properties are reported for several Ln(III) complexes of a tetradentate chelate, 5LIO-MAM, derived from the common flavor enhancer "maltol". Eu(III), Yb(III), and Nd(III) form stable ML2 complexes in aqueous solution that emit in the red
Stefano Amatori et al.
The Journal of organic chemistry, 77(5), 2207-2218 (2012-02-03)
The N,N'-bis[(3-hydroxy-4-pyron-2-yl)methyl]-N,N'-dimethylethylendiamine (malten) and 4,10-bis[(3-hydroxy-4-pyron-2-yl)methyl]-1,7-dimethyl-1,4,7,10-tetraazacyclododecane (maltonis) were synthesized and characterized. The acid-base behavior, structural characterizations, and biochemical studies in aqueous solution were reported. Each compound contains two 3-hydroxy-2-methyl-4-pyrone units (maltol) symmetrically spaced by a polyamine fragment, the 1,4-dimethylethylendiamine (malten), or
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.