D87589
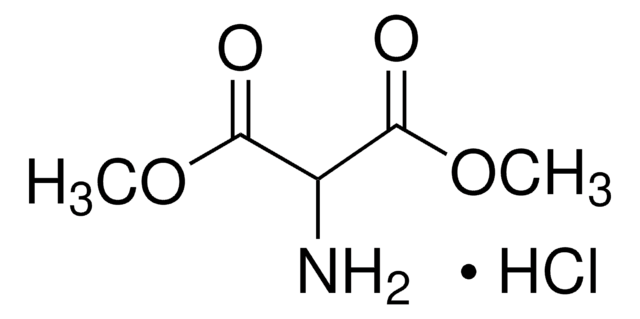
Diethyl aminomalonate hydrochloride
98%
Sinonimo/i:
Aminomalonic acid diethyl ester hydrochloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
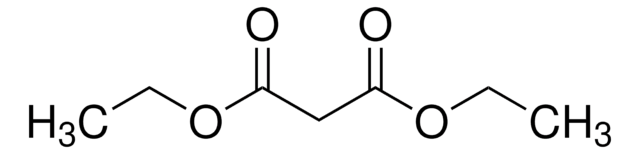
NH2CH2(COOC2H5)2 · HCl
Numero CAS:
Peso molecolare:
211.64
Beilstein:
3568037
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
crystals
Punto di fusione
165-170 °C (dec.) (lit.)
Stringa SMILE
Cl.CCOC(=O)C(N)C(=O)OCC
InChI
1S/C7H13NO4.ClH/c1-3-11-6(9)5(8)7(10)12-4-2;/h5H,3-4,8H2,1-2H3;1H
GLFVNTDRBTZJIY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
C M Metzler et al.
Biochemistry, 27(13), 4923-4933 (1988-06-28)
To establish the state of protonation of quinonoid species formed nonenzymically from pyridoxal phosphate (PLP) and diethyl aminomalonate, we have studied absorption spectra of the rapidly established steady-state mixture of species. We have evaluated the formation constant and the spectrum
Seiichi Ohta et al.
Molecular pharmaceutics, 14(9), 3105-3113 (2017-08-15)
Intraperitoneal administration of chemotherapeutics is expected for the treatment of peritoneally disseminated gastric cancer because of poor migration of the drugs from the systemic circulation to the peritoneal cavity. In this study, for intraperitoneal delivery of cisplatin (CDDP), we developed
Reaction control in the organocatalytic asymmetric one-pot, three-component reaction of aldehydes, diethyl alpha-aminomalonate and nitroalkenes: toward diversity-oriented synthesis.
Yan-Kai Liu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(32), 9873-9877 (2008-10-04)
F Hughes et al.
Organic letters, 3(18), 2911-2914 (2001-09-01)
[reaction: see text]. Nitrogen-containing tethered diacids, easily prepared by reductive alkylation of diethyl aminomalonate or ethyl cyanoglycinate, undergo double Michael reactions with 3-butyn-2-one to give highly functionalized and substituted piperidines (pipecolic acid derivatives) with surprisingly high stereoselectivity. The heterocyclic double
Charles M Blazey et al.
The Journal of organic chemistry, 67(1), 298-300 (2002-01-05)
The azomethine ylide derived from the condensation of diethyl aminomalonate with paraformaldehyde undergoes 1,3-dipolar cycloadditions with acrylate and propiolate derivatives. Contrary to a previous report, these reactions yield mixtures of regioisomers generally favoring the 2,2,3-trisubstituted product. However, the relative quantity
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.