C1930
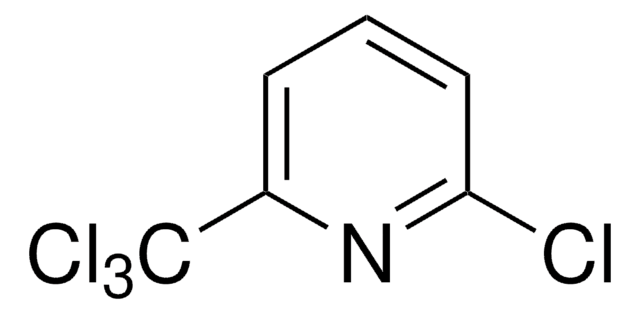
2-Chloro-6-(trichloromethyl)pyridine
≥98%
Sinonimo/i:
Nitrapyrin, 2-Chloro-6-(trichloromethyl)pyridine, CP
About This Item
Prodotti consigliati
agenzia
suitable for SM 5210
Livello qualitativo
Saggio
≥98%
Forma fisica
powder
Solubilità
ethanol: 10 mg/mL, clear, colorless to faintly yellow
Stringa SMILE
Clc1cccc(n1)C(Cl)(Cl)Cl
InChI
1S/C6H3Cl4N/c7-5-3-1-2-4(11-5)6(8,9)10/h1-3H
DCUJJWWUNKIJPH-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Biological and chemical nitrification inhibitors exhibited different effects on soil gross N nitrification rate and N(2)O production: a (15)N microcosm study.: This study examines the effects of biological and chemical nitrification inhibitors, including 2-Chloro-6-(trichloromethyl)pyridine, on soil nitrogen processes. The findings highlight the differential impacts on soil nitrogen dynamics and greenhouse gas emissions (Lan et al., 2023).
- Nitrous oxide emissions from manured soils as a function of various nitrification inhibitor rates and soil moisture contents.: This paper explores the relationship between nitrification inhibitor application rates, including 2-Chloro-6-(trichloromethyl)pyridine, and soil moisture on nitrous oxide emissions, providing insights into optimizing inhibitor use for environmental benefits (Lin and Hernandez-Ramirez, 2020).
- Nitrate losses in subsurface drainage from a corn-soybean rotation as affected by fall and spring application of nitrogen and nitrapyrin.: Investigating the seasonal application of nitrapyrin, a derivative of 2-Chloro-6-(trichloromethyl)pyridine, this study assesses its efficacy in reducing nitrate leaching and improving nitrogen use efficiency in agricultural systems (Randall and Vetsch, 2005).
- Oxidation of Nitrapyrin to 6-Chloropicolinic Acid by the Ammonia-Oxidizing Bacterium Nitrosomonas europaea.: This foundational research elucidates the microbial degradation pathway of nitrapyrin, contributing to our understanding of its environmental fate and persistence (Vannelli and Hooper, 1992).
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
212.0 °F - closed cup
Punto d’infiammabilità (°C)
100 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.