742945
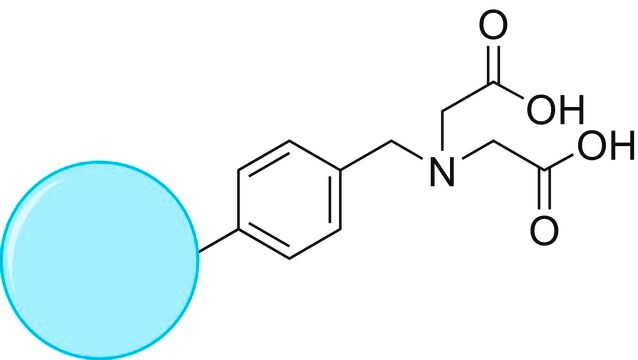
TurboBeads™ TEMPO
≥99%
Sinonimo/i:
Nano particles, magnetic, TEMPO functionalized
About This Item
Prodotti consigliati
Nome Commerciale
TurboBeads™
Saggio
≥99%
Stato
powder
Composizione
carbon content, ≤14 wt. %
Impiego in reazioni chimiche
reaction type: solution phase peptide synthesis
reactivity: alcohol reactive
Grado di funzionalizzazione
≥0.1 mmol/g loading (TEMPO)
Magnetizzazione
≥120 emu/g, mass saturation
Area superficiale
≥15 m2/g
Diametro medio
≤50 nm
Compatibilità
conforms to structure for Infrared spectrum
Applicazioni
Confezionamento
Risultati analitici
air-stability:
weight gain in air at 400°C >20 wt.%
weight gain in air at 100°C <3 wt.%
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.