69723
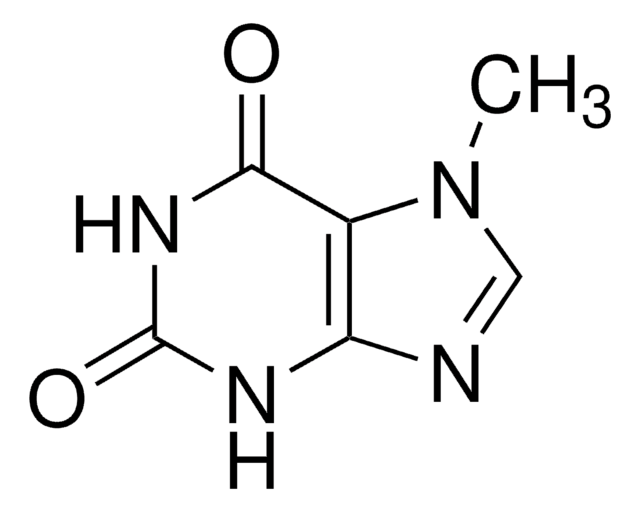
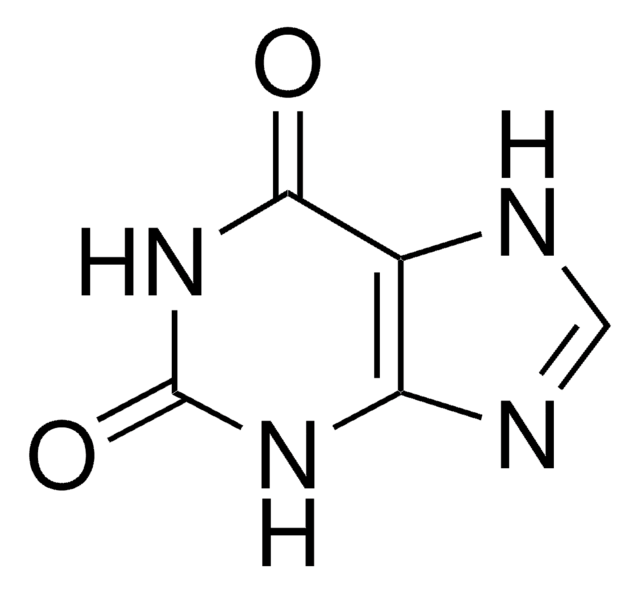
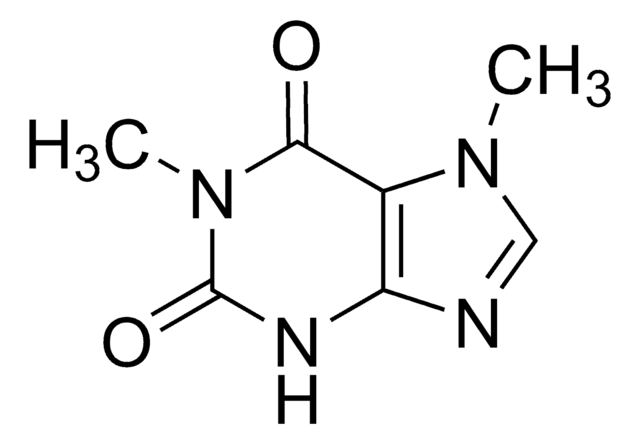
7-Methylxanthine
≥98.0% (HPLC)
Sinonimo/i:
2,6-Dihydroxy-7-methylpurine, Heteroxanthine
About This Item
Prodotti consigliati
Saggio
≥98.0% (HPLC)
Stato
powder
Punto di fusione
≥300 °C
Stringa SMILE
Cn1cnc2NC(=O)NC(=O)c12
InChI
1S/C6H6N4O2/c1-10-2-7-4-3(10)5(11)9-6(12)8-4/h2H,1H3,(H2,8,9,11,12)
PFWLFWPASULGAN-UHFFFAOYSA-N
Informazioni sul gene
rat ... Adora1(29290) , Adora2a(25369)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Confezionamento
Prodotti correlati
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Xanthine is a purine base found in most human body tissues and fluids as well as in other organisms. Methylated xanthines (methylxanthines), which include caffeine, paraxanthine, theobromine, and theophylline, commonly used for their effects as mild stiµlants and as bronchodilators, notably in the treatment of asthma symptoms. This application shows the efficient separation of several common xanthines and may be applied their analysis in any number of desired matrices.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.