678023
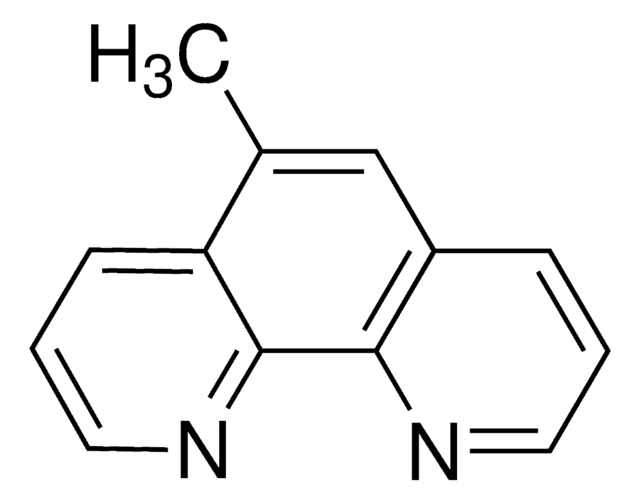
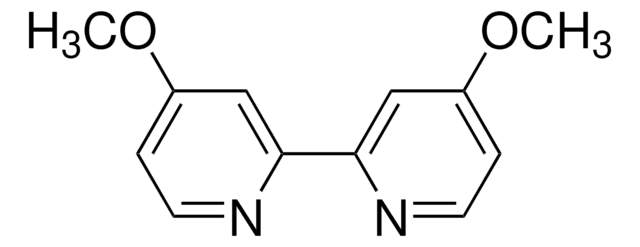
4,7-Dimethoxy-1,10-phenanthroline
97%
Sinonimo/i:
4,7-Dimethoxy-1,10-phenanthrolin
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Impiego in reazioni chimiche
reagent type: ligand
Punto di fusione
197-212 °C
Stringa SMILE
COc1ccnc2c1ccc3c(OC)ccnc23
InChI
1S/C14H12N2O2/c1-17-11-5-7-15-13-9(11)3-4-10-12(18-2)6-8-16-14(10)13/h3-8H,1-2H3
ZPGVCQYKXIQWTP-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 678023-1G | 4061826700242 |
| 678023-250MG | 4061833549773 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.





![5,8-Dimethyldibenzo[b,j][1,10]phenanthroline-6,7-diol](/deepweb/assets/sigmaaldrich/product/structures/369/172/ecac2dbe-a8f3-4161-aabb-18c93281f0e7/640/ecac2dbe-a8f3-4161-aabb-18c93281f0e7.png)