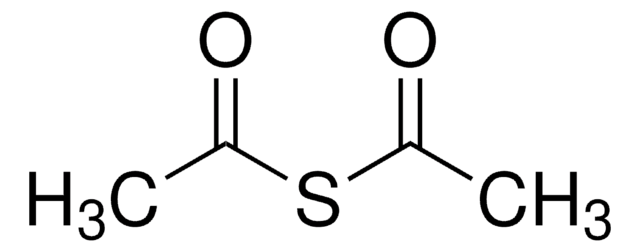
670359
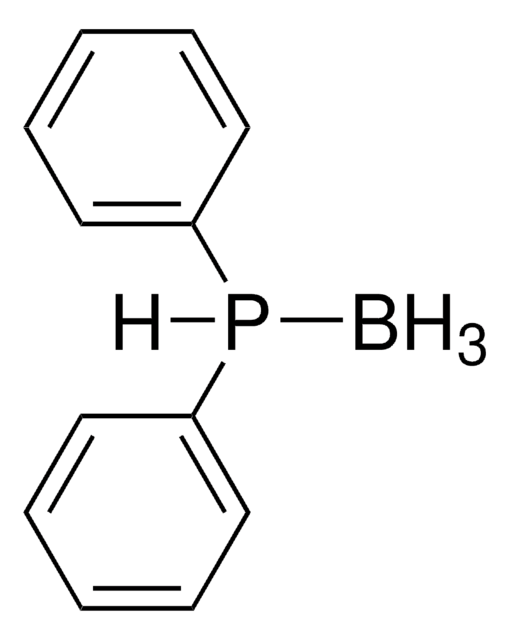
Acetylthiomethyl-diphenylphosphine borane complex
≥98.0%
Sinonimo/i:
(T-4)-[S-[(Diphenylphosphino-κP)methyl] ethanethioate]trihydroboron
About This Item
Prodotti consigliati
Saggio
≥98.0%
Stato
solid
Impiego in reazioni chimiche
reaction type: click chemistry
reagent type: ligand
reaction type: Staudinger Reaction
Punto di fusione
52-55 °C
Gruppo funzionale
phosphine
Temperatura di conservazione
2-8°C
Stringa SMILE
B.CC(=O)SCP(c1ccccc1)c2ccccc2
InChI
1S/C15H15OPS.BH3/c1-13(16)18-12-17(14-8-4-2-5-9-14)15-10-6-3-7-11-15;/h2-11H,12H2,1H3;1H3
MXPNVFCCEGQGEN-UHFFFAOYSA-N
Applicazioni
- Traceless Staudinger ligation reagent with borane protecting group.
- The borane group stabilizes the phosphine against oxidation and can be easily removed with mild basic or acidic conditions to yield the active phosphine.
- After reaction with an azide, the phosphine is eliminated in the presence of water to yield a native amide bond.
- Used in the synthesis of cyclic peptides.

Confezionamento
Note legali
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Traceless Staudinger Ligation
Based on the same working principle as the nontraceless Staudinger Ligation the auxiliary phosphine reagent can be cleaved from the product after the ligation is completed leaving a native amide bond. Thus, the total chemical synthesis of proteins and glycopeptides is enabled overcoming the limitations of native chemical ligation (NCL) of a Cys residue at the ligation juncture.
Chemoselective ligation strategies are a key success factor for chemical biology research. Ligation techniques open pathways to fully synthetic large peptides and even proteins.
The reaction between an azide and a phosphine forming an aza-ylide was discovered almost a century ago by Nobel Prize laureate Herrmann Staudinger.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.