639869
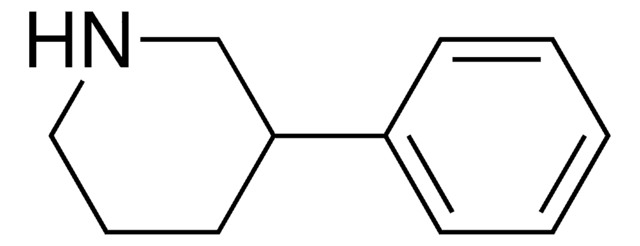
4-Phenylpiperidine
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H15N
Numero CAS:
Peso molecolare:
161.24
Beilstein:
124508
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Punto di fusione
61-65 °C (lit.)
Gruppo funzionale
phenyl
Stringa SMILE
C1CC(CCN1)c2ccccc2
InChI
1S/C11H15N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-5,11-12H,6-9H2
UTBULQCHEUWJNV-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
G H Loew et al.
Molecular pharmacology, 34(3), 363-376 (1988-09-01)
The 4-(m-OH-phenyl)piperidines are a flexible fragment of the morphine/benzomorphan fused-ring opioids. Analogs in this family were synthesized with varying 4-alkyl substituents increasing in bulk from H through methyl, n-propyl, to t-butyl, each with the three N-substituents methyl, allyl, and phenethyl.
B L Blaylock et al.
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 36(5), 1104-1113 (2011-02-04)
Although dopamine D(3) receptors have been associated with cocaine abuse, little is known about the consequences of chronic cocaine on functional activity of D(3) receptor-preferring compounds. This study examined the behavioral effects of D(3) receptor-selective 4-phenylpiperazines with differing in vitro
P Singh et al.
Journal of enzyme inhibition, 16(4), 331-338 (2002-03-28)
Two series of compounds were recently reported as novel alpha1a-selective adrenoceptor antagonists. In the first series, a dihydropyrimidone moiety is attached to a 4-phenyl piperidine containing side chain, while in the second, it is linked to a 4-substituted phenyl piperazine
Xing-hai Wang et al.
European journal of medicinal chemistry, 41(2), 226-232 (2006-01-13)
A nonlinear QSAR study was conducted on a series of 4-phenylpiperidine derivatives (4PPs) acting as mu opioid agonists by three-layer back-propagation neural network (NN) method. At first a variety of molecular descriptors were calculated and then selected with two-stage least
Karel Vervisch et al.
Organic & biomolecular chemistry, 10(16), 3308-3314 (2012-03-14)
Non-activated 2-(4-chloro-2-cyano-2-phenylbutyl)aziridines were used as building blocks for the stereoselective synthesis of novel cis-2-cyanomethyl-4-phenylpiperidines via a microwave-assisted aziridine to piperidine ring expansion followed by a radical-induced nitrile translocation through initial formation and subsequent cleavage of intermediate bicyclic iminyl radicals. Furthermore
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 639869-5G | 4061833046104 |
| 639869-1G | 4061826291900 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.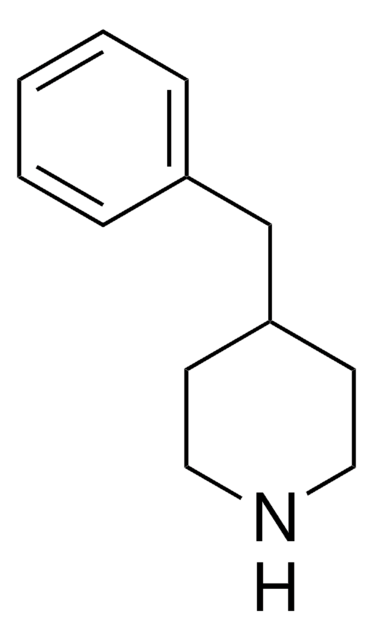

![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)

![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)