638439
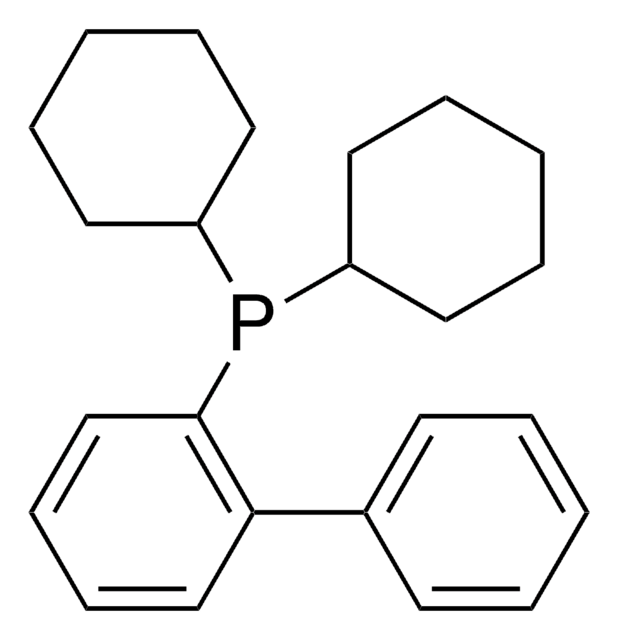
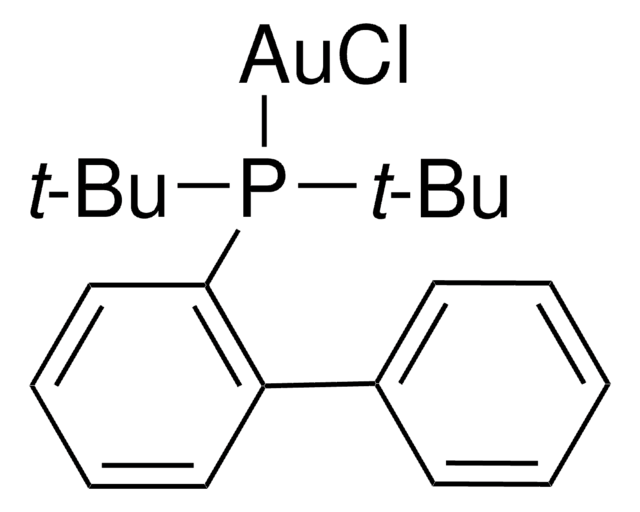
(2-Biphenyl)di-tert-butylphosphine
97%
Sinonimo/i:
(2-Biphenylyl)di-tert-butylphosphine
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Impiego in reazioni chimiche
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-X Bond Formation
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
Punto di fusione
86-88 °C (lit.)
Gruppo funzionale
phosphine
Stringa SMILE
CC(C)(C)P(c1ccccc1-c2ccccc2)C(C)(C)C
InChI
1S/C20H27P/c1-19(2,3)21(20(4,5)6)18-15-11-10-14-17(18)16-12-8-7-9-13-16/h7-15H,1-6H3
CNXMDTWQWLGCPE-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Learn more about Buchwald Phosphine Ligands
Applicazioni
- Hydrophenoxylation of unactivated internal alkynes.
- Microwave-mediated Suzuki-Miyaura cross-coupling of benzylic bromides.
- Pharmaceutical synthesis of novel imidazo[1,2-a]pyridines, having potent activity against the herpes virus.
- Barluenga′s coupling of vinyl bromides with hydrazines.
- Pd-catalyzed 2,3-diarylation of α,α-disubstituted-3-thiophenemethanols, via cleavage of C-H and C-C bonds.
Catalyst for:
- Decarboxylative cross-coupling of dialkoxybenzoic acids with diaryl disulfides or diaryl diselenides
- Stereoselective preparation of imidazolidinones via intramolecular hydroamination of N-allylic-N-arylureas
- Regioselective arylation of olefins with aryl chlorides
- Cross-coupling reaction for the synthesis of polyunsaturated macrolactones
- Regioselective O-alkylation reactions
- Sonogashira-type cross coupling
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Buchwald Ligands
Buchwald Phosphine Ligands
Over the past several years, Pd-catalyzed cross-coupling of silicon compounds has rapidly gained acceptance as a suitable alternative to more commonly known methods such as: Stille (Sn), Kumada (Mg), Suzuki (B), and Negishi (Zn) cross-couplings.
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.