57410
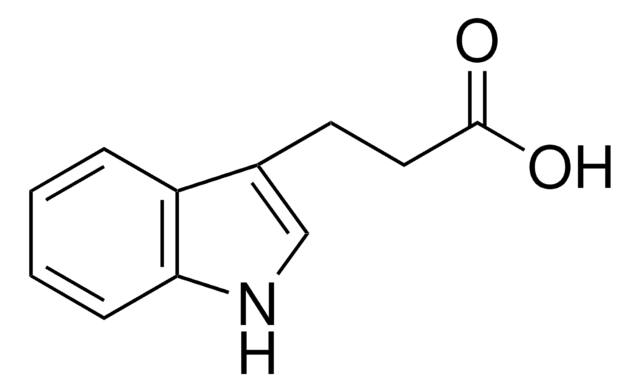
Indole-3-propionic acid
≥97.0% (T)
Sinonimo/i:
NSC 3252, NSC 47831, 3-(3-Indolyl)propanoic acid, 3-(3-Indolyl)propionic acid, IPA
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H11NO2
Numero CAS:
Peso molecolare:
189.21
Beilstein:
147733
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥97.0% (T)
Forma fisica
solid
Stringa SMILE
OC(=O)CCc1c[nH]c2ccccc12
InChI
1S/C11H11NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2,(H,13,14)
GOLXRNDWAUTYKT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Indole-3-propionic acid can be obtained from tryptophan by deamination reaction.
Applicazioni
Reactant for preparation of:
- Fluorescent analogues of strigolactones
- Anti-tumor agents
- Melanocortin receptors ligands
- Immunosuppressive agents
- Iinhibitors of hepatitis C virus
- Histamine H4 receptor agonists
- NR2B/NMDA receptor antagonists
- CB1 antagonist for the treatment of obesity
- Antibacterial agents
- Inhibitor of TGF-β receptor binding
Azioni biochim/fisiol
Studied as an adjunct to improve perfusion after liver transplant.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Wanguo Wei et al.
Bioorganic & medicinal chemistry letters, 19(24), 6926-6930 (2009-11-10)
New small molecule inhibitors of HCV were discovered by screening a small library of indoline alkaloid-type compounds. An automated assay format was employed which allowed identification of dimerization inhibitors of core, the capsid protein of the virus. These compounds were
Francis Giraud et al.
Bioorganic & medicinal chemistry letters, 20(17), 5203-5206 (2010-07-27)
N-aryl-3-(indol-3-yl)propanamides were synthesized and their immunosuppressive activities were evaluated. This study highlighted the promising potency of 3-[1-(4-chlorobenzyl)-1H-indol-3-yl]-N-(4-nitrophenyl)propanamide 15 which exhibited a significant inhibitory activity on murine splenocytes proliferation assay in vitro and on mice delayed-type hypersensitivity (DTH) assay in vivo.
Ragan, J. A.; et al.
Organic Process Research & Development, 13, 186-186 (2009)
Peng Xu et al.
Bioorganic & medicinal chemistry letters, 17(12), 3330-3334 (2007-04-27)
A series of novel acylide derivatives have been synthesized from erythromycin A via a facile procedure. By applying this procedure, cyclic carbonation to C-11,12 position, acylation to C-3 hydroxyl, and deprotection provided the desired acylides. These compounds showed antibacterial activity
Rosaria Gitto et al.
Bioorganic & medicinal chemistry, 17(4), 1640-1647 (2009-01-23)
A combined ligand-based and structure-based approach has previously allowed us to identify NR2B/NMDA receptor antagonists containing indole scaffold. In order to further explore the main structure activity relationships of this class of derivatives we herein report the design, synthesis and
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.