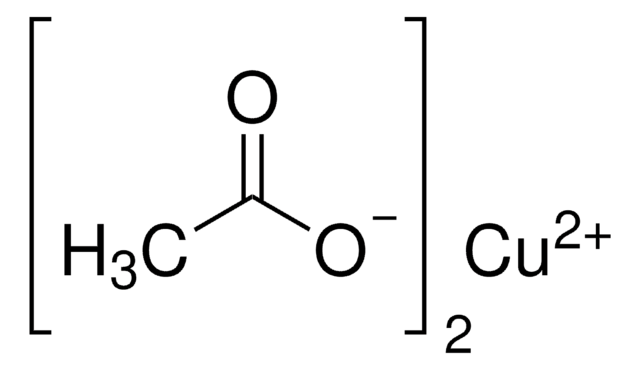
539406
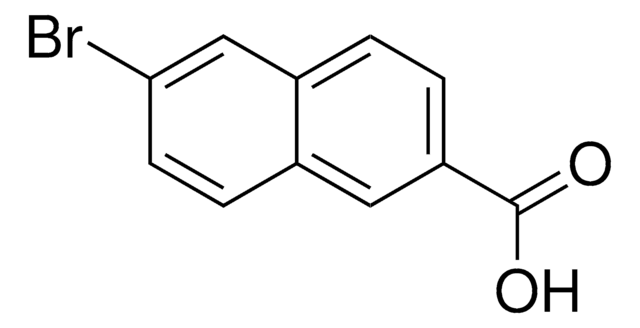
Methyl 6-bromo-2-naphthoate
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
BrC10H6CO2CH3
Numero CAS:
Peso molecolare:
265.10
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
123-126 °C (lit.)
Gruppo funzionale
bromo
ester
Stringa SMILE
COC(=O)c1ccc2cc(Br)ccc2c1
InChI
1S/C12H9BrO2/c1-15-12(14)10-3-2-9-7-11(13)5-4-8(9)6-10/h2-7H,1H3
JEUBRLPXJZOGPX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Methyl 6-bromo-2-naphthoate undergoes aromatic Finkelstein reaction followed by hydrolysis to afford 6-iodo-2-naphthoic acid.
Applicazioni
Methyl 6-bromo-2-naphthoate may be used to synthesize:
- 6-vinyl-2-naphthalencarbaldehyde
- methyl 6-(3-tert-butyl-4-methoxyphenyl)-2-naphthoate
- methyl 6-[3-tert-butyl-4-[(tert-butyldiethylsilyl)oxy]-phenyl]-2-naphthoate
- methyl 6-[3-(1-adamantyl)-4-[(tert-butyldimethylsilyl)-oxy]phenyl]-2-naphthoate
- methyl 6-[3-(1-adamantyl)-4-[[(2,3-dimethyl-1,3-dioxolan-4-yl)methylloxy]phenyl]-2-naphthoate
- 2-bromo-6-(bromomethyl)naphthalene
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
B Charpentier et al.
Journal of medicinal chemistry, 38(26), 4993-5006 (1995-12-22)
The retinoic acid receptors (RARs) transduce retinoid dependant gene regulation, and many biological effects of retinoids are mediated through binding and activation of three closely related receptor subtypes (RAR alpha, RAR beta, and RAR gamma). In order to investigate the
Carboxy-1, 4-phenylenevinylene-and carboxy-2, 6-naphthylene-vinylene unsymmetrical substituted zinc phthalocyanines for dye-sensitized solar cells.
Silvestri F, et al.
Journal of Porphyrins and Phthalocyanines, 13(03), 369-375 (2009)
Phil M Pithan et al.
Beilstein journal of organic chemistry, 12, 854-862 (2016-06-25)
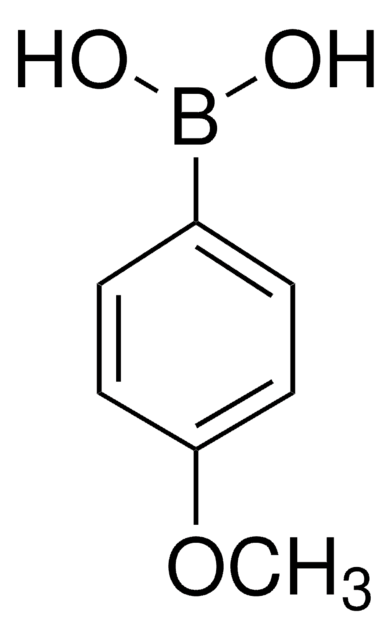
Cationic biaryl derivatives were synthesized by Suzuki-Miyaura coupling of 3-bromonaphtho[1,2-b]quinolizinium bromide with arylboronic acids. The resulting cationic biaryl derivatives exhibit pronounced fluorosolvatochromic properties. First photophysical studies in different solvents showed that the emission energy of the biaryl derivatives decreases with
Mark W Irvine et al.
Journal of medicinal chemistry, 55(1), 327-341 (2011-11-25)
Competitive N-methyl-d-aspartate receptor (NMDAR) antagonists bind to the GluN2 subunit, of which there are four types (GluN2A-D). We report that some N(1)-substituted derivatives of cis-piperazine-2,3-dicarboxylic acid display improved relative affinity for GluN2C and GluN2D versus GluN2A and GluN2B. These derivatives
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)