522910
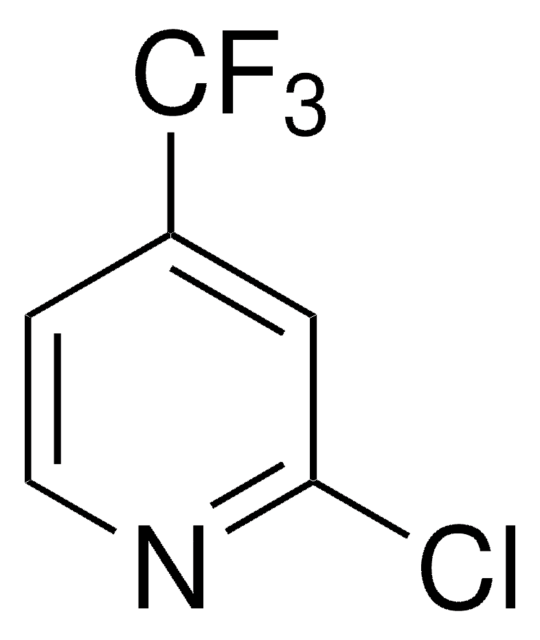
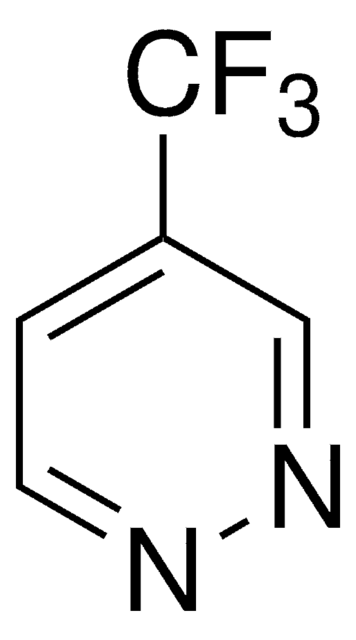
4-(Trifluoromethyl)pyridine
97%
Sinonimo/i:
4-(Trifluoromethyl)pyridine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H4F3N
Numero CAS:
Peso molecolare:
147.10
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Indice di rifrazione
n20/D 1.417 (lit.)
P. ebollizione
110 °C (lit.)
Densità
1.27 g/mL at 25 °C (lit.)
Gruppo funzionale
fluoro
Stringa SMILE
FC(F)(F)c1ccncc1
InChI
1S/C6H4F3N/c7-6(8,9)5-1-3-10-4-2-5/h1-4H
IIYVNMXPYWIJBL-UHFFFAOYSA-N
Descrizione generale
4-(Trifluoromethyl)pyridine is a pyridine derivative. It can be prepared by trifluoromethylation of 4-iodobenzene.
Applicazioni
4-(Trifluoromethyl)pyridine may be used in the following:
- Preparation of (trifluoromethyl)pyridyllithiums, via metalation reaction.
- Synthesis of metal-organic frameworks (MOFs).
- Synthesis of methiodide salts.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
68.0 °F - closed cup
Punto d’infiammabilità (°C)
20 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Manfred Schlosser et al.
Chemical Society reviews, 36(7), 1161-1172 (2007-06-20)
Pyridines carrying heterosubstituents (such as carboxy, amido, amino, alkoxy or trifluoromethyl groups or solely individual halogen atoms) can be readily and site selectively metalated. Subsequent reaction with a suitable electrophile opens rational access to a wealth of new building blocks
Fluorinated pyridine derivatives: Part 1. The synthesis of some mono-and bis-quaternary pyridine salts of potential use in the treatment of nerve agent poisoning.
Timperley CM, et al.
Journal of Fluorine Chemistry, 126(8), 1160-1165 (2005)
Enhancement of CO2/N2 selectivity in a metal-organic framework by cavity modification.
Bae YS, et al.
Journal of Materials Chemistry, 19(15), 2131-2134 (2009)
Copper-mediated trifluoromethylation of heteroaromatic compounds by trifluoromethyl sulfonium salts.
Cheng-Pan Zhang et al.
Angewandte Chemie (International ed. in English), 50(8), 1896-1900 (2011-02-18)
Malcolm E Tessensohn et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 18(16), 2250-2257 (2017-06-14)
The voltammetric behavior of 2,3,5,6-tetramethyl-1,4-phenylenediamine was found to be able to differentiate the hydrogen acceptor abilities of electroinactive pyridine compounds in acetonitrile. Weak and strong hydrogen acceptors were distinguished through the onset of a third oxidation process that came about
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.