446424
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol
97%
Sinonimo/i:
3,5-Di-tert-butyl-4-hydroxyphenylmethanol, 4-Hydroxymethyl-2,6-di-tert-butylphenol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HOC6H2[C(CH3)3]2CH2OH
Numero CAS:
Peso molecolare:
236.35
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Punto di fusione
139-141 °C (lit.)
Gruppo funzionale
hydroxyl
Stringa SMILE
CC(C)(C)c1cc(CO)cc(c1O)C(C)(C)C
InChI
1S/C15H24O2/c1-14(2,3)11-7-10(9-16)8-12(13(11)17)15(4,5)6/h7-8,16-17H,9H2,1-6H3
HNURKXXMYARGAY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol can be used as a reactant to synthesize:
- 2,6-di-tert-butyl-4-(dodecylselanylmethyl)phenol and bis(3,5-di-tert-butyl-4-hydroxybenzyl) selenide by reacting with dodecaneselenolate and sodium selenide.
- Monomeric antioxidant by reacting with imidazole and N-[4-(chlorocarbonyl)phenyl]maleimide.
- Sulfur-containing butylated hydroxytoluene derivatives by reacting with aryl/alky dithiols.
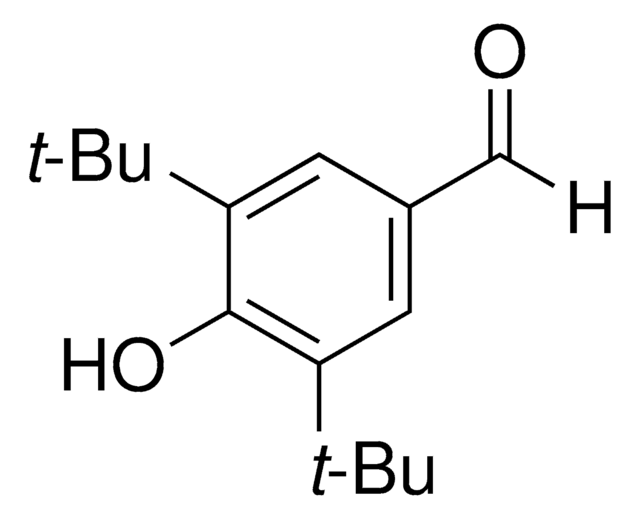
- 3,5-Di-tert-butyl-4-hydroxybenzaldehyde by oxidation reaction using stabilized IBX.
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 3
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
O L Brekke et al.
Cytokine, 4(4), 269-280 (1992-07-01)
The effect of commonly used food antioxidants on recombinant tumor necrosis factor alpha (rTNF-alpha)-induced cytotoxicity, growth enhancement and adhesion has been evaluated. Butylated hydroxyanisole (BHA) and 4-hydroxymethyl-2,6-di-t-butylphenol (HBP) were the only two of nine antioxidants that completely inhibited rTNF-alpha-induced cytotoxicity
L R Barclay et al.
Biochimica et biophysica acta, 1328(1), 1-12 (1997-08-14)
Phenolic antioxidants of the hydroxychroman class, alpha-tocopherol (alpha-TOC) and 2,2,5,6,7-pentamethyl-6-hydroxychroman (PMHC), and the hindered phenols 2,3-dihydro-5-hydroxy-2,2,4-trimethylnaphtho[1,2-b]furan (NFUR), 2,6-di-tert-butyl-4-methoxyphenol (DBHA), and 2,6-di-tert-butyl-4-methyl phenol (BHT), were delivered into oxidizable (ACCEPTOR) liposomes of dilinoleoylphosphatidylcholine (DLPC) or 1-palmitoyl-2-linoleoyl-phosphatidylcholine (PLPC) from saturated DONOR liposomes of
REACTION OF SEVEN-AND EIGHT-MEMBERED CYCLIC PHOSPHOROCHLORIDITES WITH 3, 5-DI-tert-BUTYL-4-HYDROXYBENZYL ALCOHOL: FACILE P [sbnd] C BOND FORMATION.
Odorisio PA, et al.
Phosph. Sulfur Relat. Elem., 20(3), 273-277 (1984)
The antioxidant activity of 3, 5-di-tert-butyl-4-hydroxybenzyl derivatives.
Kim DH and Kummerow FA.
Journal of the American Chemical Society, 39(3), 150-155 (1962)
Synthesis of new polymeric antioxidants.
Oh DR, et al.
Bull. Korean Chem. Soc., 22(6), 629-632 (2001)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.