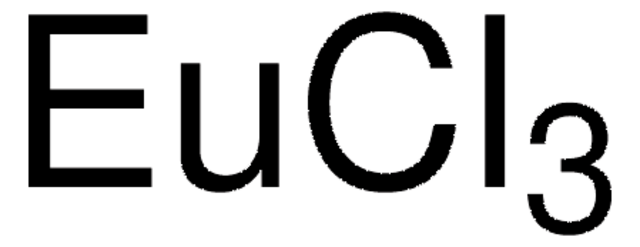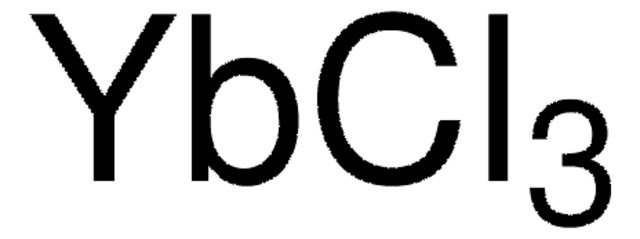429732
Europium(III) chloride
anhydrous, powder, 99.99% trace metals basis
Sinonimo/i:
Europium trichloride
About This Item
Prodotti consigliati
Grado
anhydrous
Livello qualitativo
Saggio
99.99% trace metals basis
Stato
powder
Impiego in reazioni chimiche
reagent type: catalyst
core: europium
Impurezze
≤150.0 ppm Trace Rare Earth Analysis
Punto di fusione
850 °C (lit.)
Densità
4.89 g/mL at 25 °C (lit.)
Stringa SMILE
Cl[Eu](Cl)Cl
InChI
1S/3ClH.Eu/h3*1H;/q;;;+3/p-3
NNMXSTWQJRPBJZ-UHFFFAOYSA-K
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Photochemical Recovery of Europium from Non-aqueous Solutions: This study explores the photochemical processes involving Europium(III) chloride, which are significant for applications in material recovery and recycling (B Van den Bogaert, L Gheeraert, 2016).
- Electrochemistry of Europium(III) Chloride in Various Eutectics: Investigation of the electrochemical properties of Europium(III) chloride in different molten salt mixtures, important for developing new materials and nuclear applications (CA Schroll, S Chatterjee, TG Levitskaia, 2017).
Accessorio
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Thermoelectric Performance of Perovskite-type Oxide Materials
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 429732-1G | 4061832107707 |
| 429732-5G | 4061833424926 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.