366765
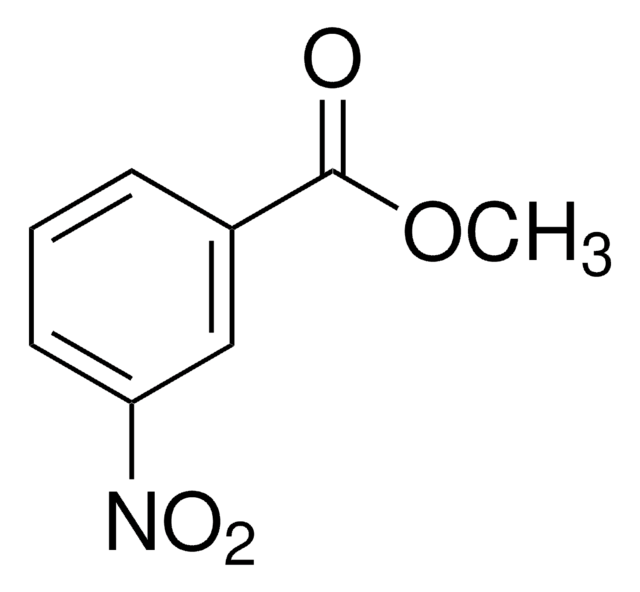
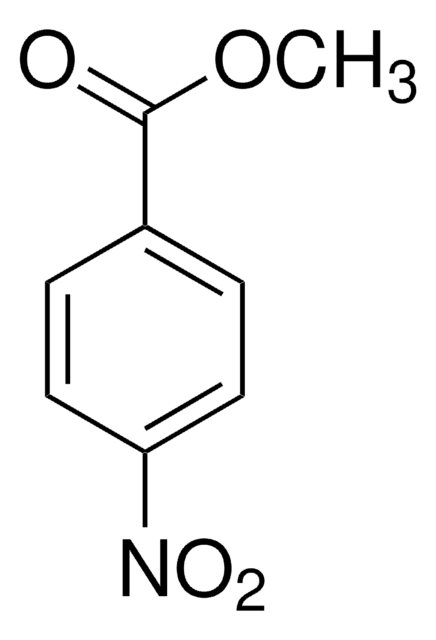
Methyl 2-methyl-3-nitrobenzoate
97%
Sinonimo/i:
Methyl 3-nitro-o-toluate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3C6H3(NO2)CO2CH3
Numero CAS:
Peso molecolare:
195.17
Beilstein:
1964020
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Forma fisica
solid
Punto di fusione
62-65 °C (lit.)
Gruppo funzionale
ester
nitro
Stringa SMILE
COC(=O)c1cccc(c1C)[N+]([O-])=O
InChI
1S/C9H9NO4/c1-6-7(9(11)14-2)4-3-5-8(6)10(12)13/h3-5H,1-2H3
CRZGFIMLHZTLGT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Methyl 2-methyl-3-nitrobenzoate may be used in the synthesis of:
- methyl indole-4-carboxylate
- 5-aminoisoquinolin-1(2H)-one
- 5-nitroisocoumarin
- substituted nitrostyrene benzoic acids, via reaction with aromatic aldehydes in the presence of DBU in DMSO
- 4-(hydroxymethyl)-1-tosylindole, via Batcho-Leimgruber modification of the Reissert indole synthesis
- [2-(4-fluorophenyl)-1H-indol-4-yl]-1-pyrrolidinylmethanone
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Synthesis of 2-Arylindole-4-Carboxylic Amides:[2-(4-Fluorophenyl)-1H-Indol-4-YL]-1-Pyrrolidinylmethanone.
Kuethe JT and Beutner GL.
Organic Syntheses, 92-104 (2009)
A N Brubaker et al.
Journal of medicinal chemistry, 29(8), 1528-1531 (1986-08-01)
Three 7-methyl-1,4-dioxa-7-azaspiro[4.5]decanes that contained either the benzyl, 3-indolylmethyl, or 4-indolylmethyl group at the 6-position were synthesized via alkylation of the pyrrolidine enamine of the key intermediate, ethyl 3-oxopiperidine-1-carboxylate. The spirodecane derivatives were evaluated for in vivo central and peripheral dopamine
The Chemistry of Indoles. XII. A Facile Route to 5-Nitroisocoumarins and Methyl Indole-4-carboxylate.
<BIG>Masanori S, et al. </BIG>
Chemical & Pharmaceutical Bulletin, 29(1), 249-253 (1981)
Preparation of 2-arylindole-4-carboxylic amide derivatives.
Kuethe JT and Davies LW.
Tetrahedron, 62(49), 11381-11390 (2006)
M C McDonald et al.
British journal of pharmacology, 130(4), 843-850 (2000-06-24)
Poly (ADP-ribose) synthetase (PARP) is a nuclear enzyme activated by strand breaks in DNA, which are caused inter alia by reactive oxygen species (ROS). Here we report on (i) a new synthesis of a water-soluble and potent PARP inhibitor, 5-aminoisoquinolinone
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.