360910
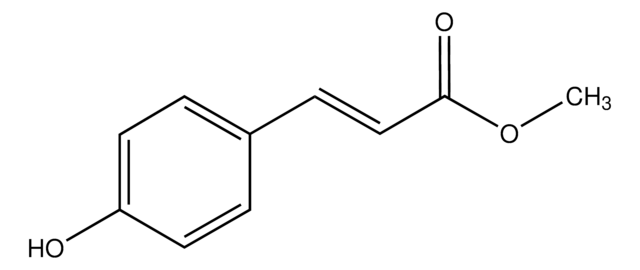
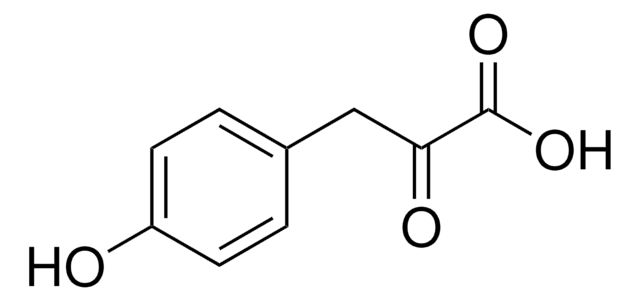
Methyl 3-(4-hydroxyphenyl)propionate
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
HOC6H4CH2CH2CO2CH3
Numero CAS:
Peso molecolare:
180.20
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
P. ebollizione
108 °C/11 mmHg (lit.)
Punto di fusione
39-41 °C (lit.)
Gruppo funzionale
ester
Stringa SMILE
COC(=O)CCc1ccc(O)cc1
InChI
1S/C10H12O3/c1-13-10(12)7-4-8-2-5-9(11)6-3-8/h2-3,5-6,11H,4,7H2,1H3
XRAMJHXWXCMGJM-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
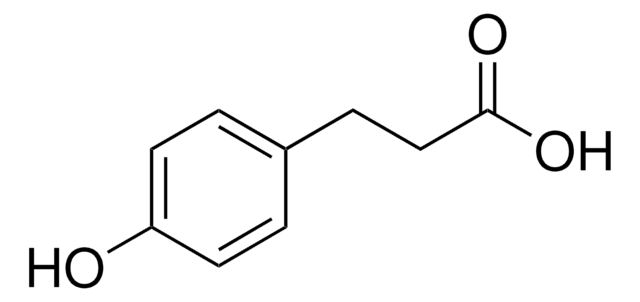
Methyl 3-(4-hydroxyphenyl)propionate is reported to be responsible for biological nitrification inhibition in sorghum (Sorghum bicolor).
Applicazioni
Methyl 3-(4-hydroxyphenyl)propionate may be used in the enzymatic coupling of saccharides to protein.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Ruud ter Haar et al.
Carbohydrate research, 346(8), 1005-1012 (2011-04-14)
To enable enzymatic coupling of saccharides to proteins, several di- and trisaccharides were hydroxy-arylated using anhydrous transesterification with methyl 3-(4-hydroxyphenyl)propionate, catalyzed by potassium carbonate. This transesterification resulted in the attachment of up to 3 hydroxy-aryl units per oligosaccharide molecule, with
Hossain A K M Zakir et al.
The New phytologist, 180(2), 442-451 (2008-07-29)
Nitrification results in poor nitrogen (N) recovery and negative environmental impacts in most agricultural systems. Some plant species release secondary metabolites from their roots that inhibit nitrification, a phenomenon known as biological nitrification inhibition (BNI). Here, we attempt to characterize
Alexander K Andrianov et al.
Biomacromolecules, 19(8), 3467-3478 (2018-06-29)
Novel oppositely charged polyphosphazene polyelectrolytes containing grafted poly(ethylene glycol) (PEG) chains were synthesized as modular components for the assembly of biodegradable PEGylated protein delivery vehicles. These macromolecular counterparts, which contained either carboxylic acid or tertiary amino groups, were then formulated
Andre P Martinez et al.
Biomacromolecules, 18(6), 2000-2011 (2017-05-20)
A series of biodegradable drug delivery polymers with intrinsic multifunctionality have been designed and synthesized utilizing a polyphosphazene macromolecular engineering approach. Novel water-soluble polymers, which contain carboxylic acid and pyrrolidone moieties attached to an inorganic phosphorus-nitrogen backbone, were characterized by
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 360910-5G | 4061831810677 |
| 360910-25G |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.