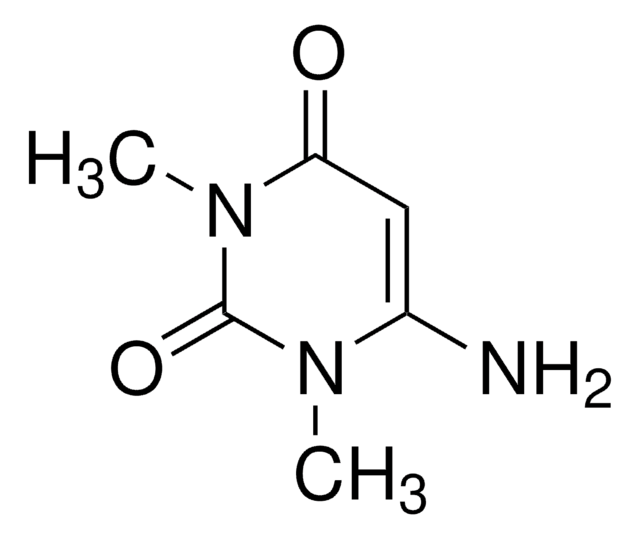
349801
1,3-Dimethyluracil
99%
Sinonimo/i:
1,3-Dimethyl-2,4(1H,3H)-pyrimidinedione, 2,4-Dihydroxy-1,3-dimethylpyrimidine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H8N2O2
Numero CAS:
Peso molecolare:
140.14
Beilstein:
124074
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Forma fisica
powder
Punto di fusione
119-122 °C (lit.)
Stringa SMILE
CN1C=CC(=O)N(C)C1=O
InChI
1S/C6H8N2O2/c1-7-4-3-5(9)8(2)6(7)10/h3-4H,1-2H3
JSDBKAHWADVXFU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
1,3-Dimethyluracil is a pyrimidine derivative. Stability of the C6-centered carbanions derived from 1,3-dimethyluracil has been investigated in the gas phase and in DMSO and water solutions. The excited state structural dynamics of 1,3-dimethyluracil (DMU) in water and acetonitrile has been studied by resonance Raman spectroscopy. Crystal structure of 1,3-dimethyluracil has been reported. Ultraviolet irradiation of aqueous 1,3-dimethyluracil results in hydration of the 5:6 double bond of the uracil ring to form 1,3-dimethyl-6-oxy-hydrouracil.
Applicazioni
1,3-Dimethyluracil is suitable reagent used to investigate the steady-state absorption and fluorescence spectra of uracil derivatives. It may be used in the preparation of 2,6-dihydroxynicotinamide.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Adam Gryff-Keller et al.
The journal of physical chemistry. A, 116(39), 9632-9638 (2012-09-14)
The practical utility of the method of retrieving the relaxation rate of a quadrupole nucleus via the scalar relaxation of the second kind (SC2) of an I = 1/2 spin nucleus has been considered once again. The study was motivated
Nicholas A Senger et al.
Tetrahedron, 69(26), 5287-5292 (2013-09-28)
The stabilities of the C6-centered carbanions derived from 1,3-dimethyluracil, N-methyl-2-pyridone, and N-methyl-4-pyridone were systematically investigated in the gas phase and in DMSO and water solutions. The stabilities of the carbanions in the gas phase and DMSO were directly measured through
Probing noncovalent interactions in biomolecular crystals with terahertz spectroscopy.
Thomas Kleine-Ostmann et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 9(4), 544-547 (2008-02-15)
H P Schuchmann et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 50(6), 1051-1068 (1986-12-01)
Hydroxymethyl radicals .CH2OH, generated by the radiolysis of methanol (0.5 mol dm-3) in N2O-saturated aqueous solutions, were reacted with 1,3-dimethyluracil or 1,3-dimethylthymine (10(-3) mol dm-3). The products were identified and their G values determined. It has been concluded that in
Anna A Zadorozhnaya et al.
The journal of physical chemistry. A, 114(4), 2001-2009 (2010-01-09)
The electronic structure of 1,3-dimethyluracil and its dimer is characterized by ab initio calculations. The methylation eliminates the H-bonded isomers and allows one to focus on the pi-stacked manifold. In the neutral species, methylation increases the binding energy by 3-4
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.