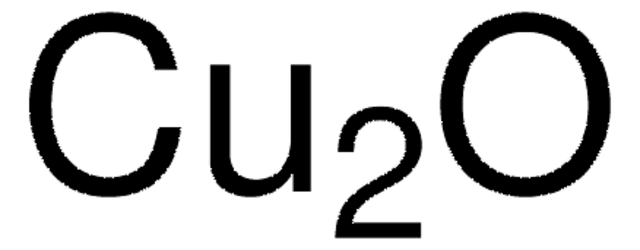229628
Copper(I) chloride
≥99.995% trace metals basis
Sinonimo/i:
Copper monochloride, Cuprous chloride
About This Item
Prodotti consigliati
Grado
for analytical purposes
Livello qualitativo
Tensione di vapore
1.3 mmHg ( 546 °C)
Saggio
≥99.995% trace metals basis
Stato
powder
Impiego in reazioni chimiche
reagent type: catalyst
core: copper
tecniche
mass spectrometry (MS): suitable
Impurezze
≤50.0 ppm Trace Rare Earth Analysis
P. ebollizione
1490 °C (lit.)
Punto di fusione
430 °C (lit.)
Solubilità
slightly soluble 0.47 g/L at 20 °C
applicazioni
battery manufacturing
Stringa SMILE
Cl[Cu]
InChI
1S/ClH.Cu/h1H;/q;+1/p-1
OXBLHERUFWYNTN-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Shows unique character as an initiator of radical reactions such as the hydrostannation of α,β-unsaturated ketones.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
Thermoelectric Performance of Perovskite-type Oxide Materials
Spectral conversion for solar cells is an emerging concept in the field of photovoltaics, and it has the potential to increase significantly the efficiency of solar cells. Lanthanide ions are ideal candidates for spectral conversion, due to their high luminescence efficiencies and rich energy level structure that allows for great flexibility in the upconversion and downconversion of photons in a wide spectral region (NIR-VIS-UV).
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Protocolli
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 229628-100G | 4061833595718 |
| 229628-10G | 4061838781963 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.