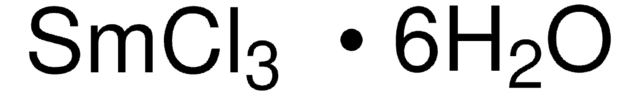228931
Cerium(III) chloride heptahydrate
99.9% trace metals basis
Sinonimo/i:
Cerous chloride heptahydrate
About This Item
Prodotti consigliati
Saggio
99.9% trace metals basis
Forma fisica
crystals and lumps
Impiego in reazioni chimiche
reagent type: catalyst
core: cerium
Impurezze
≤1500.0 ppm Trace Rare Earth Analysis
Densità
~3.94 g/mL at 25 °C (lit.)
Stringa SMILE
[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].Cl[Ce](Cl)Cl
InChI
1S/Ce.3ClH.7H2O/h;3*1H;7*1H2/q+3;;;;;;;;;;/p-3
KPZSTOVTJYRDIO-UHFFFAOYSA-K
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- As a precursor to prepare cerium oxide nanoparticles for biomedical applications and photocatalytic degradation.
- As a solution to fabricate thin films of CeO2 on glass substrates by the spray pyrolysis process.
- As a dopant to fabricate ZnO and CeO2 nanocrystals for electrochemical sensing of H2O2 and photocatalytic degradation of Rhodamine B and Congo red dyes.
- As an additive to prepare corrosion-inhibiting formulations and coatings.
- To synthesize carbon nanofiber composites to fabricate high-temperature polymer electrolyte membrane fuel cell cathodes.
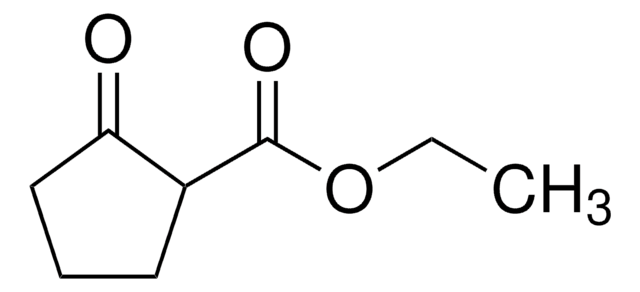
- As a support for the combination of cerium(III)chloride heptahydrate and sodium iodide supported on silica gel to promoteMichael-type additions. These catalysts are used to convert from indolesand nitroalkenes to 2-indolyl-1-nitroalkane derivatives in good yields.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.