224529
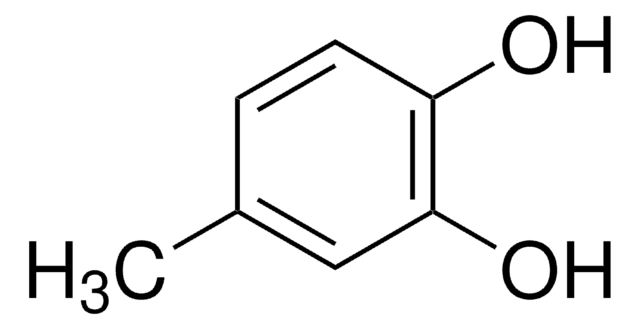
3-Chloro-4-hydroxyphenylacetic acid
99%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
ClC6H3(OH)CH2CO2H
Numero CAS:
Peso molecolare:
186.59
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Forma fisica
powder
Punto di fusione
108-110 °C (lit.)
Gruppo funzionale
carboxylic acid
chloro
Stringa SMILE
OC(=O)Cc1ccc(O)c(Cl)c1
InChI
1S/C8H7ClO3/c9-6-3-5(4-8(11)12)1-2-7(6)10/h1-3,10H,4H2,(H,11,12)
IYTUKSIOQKTZEG-UHFFFAOYSA-N
Descrizione generale
3-Chloro-4-hydroxyphenylacetic acid is an auxin influx inhibitor. It is one of the major chlorinated metabolite of chlorotyrosine.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Petr Hosek et al.
Journal of experimental botany, 63(10), 3815-3827 (2012-03-23)
The molecular basis of cellular auxin transport is still not fully understood. Although a number of carriers have been identified and proved to be involved in auxin transport, their regulation and possible activity of as yet unknown transporters remain unclear.
Ali R Mani et al.
The Journal of biological chemistry, 282(40), 29114-29121 (2007-08-10)
During inflammation, neutrophil- and monocyte-derived myeloperoxidase catalyzes the formation of hypochlorous acid, which can chlorinate tyrosine residues in proteins to form chlorotyrosine. However, little is known of the metabolism and disposition of chlorotyrosine in vivo. Following infusion of deuterium-labeled [D(4)]chlorotyrosine
H Nguyen et al.
Toxicology, 160(1-3), 207-217 (2001-03-14)
Exposure to airborne pollutants such as tobacco smoke is associated with increased activation of inflammatory-immune processes and is thought to contribute to the incidence of respiratory tract disease. We hypothezised that cigarette smoke (CS) could synergize with activated inflammatory/immune cells
B A van de Pas et al.
Applied and environmental microbiology, 67(9), 3958-3963 (2001-08-30)
The amount of energy that can be conserved via halorespiration by Desulfitobacterium dehalogenans JW/IU-DC1 was determined by comparison of the growth yields of cells grown with 3-chloro-4-hydroxyphenyl acetate (Cl-OHPA) and different electron donors. Cultures that were grown with lactate, pyruvate
I Utkin et al.
Applied and environmental microbiology, 61(1), 346-351 (1995-01-01)
Resting cells of Desulfitobacterium dehalogenans JW/IU-DC1 growth with pyruvate and 3-chloro-4-hydroxyphenylacetate (3-Cl-4-OHPA) as the electron acceptor and inducer of dehalogenation reductively ortho-dehalogenate pentachlorophenol (PCP); tetrachlorophenols (TeCPs); the trichlorophenols 2,3,4-TCP, 2,3,6-TCP, and 2,4,6-TCP; the dichlorophenols 2,3-DCP, 2,4-DCP, and 2,6-DCP; 2,6-dichloro-4-R-phenols (2,6-DCl-4-RPs
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.