223220
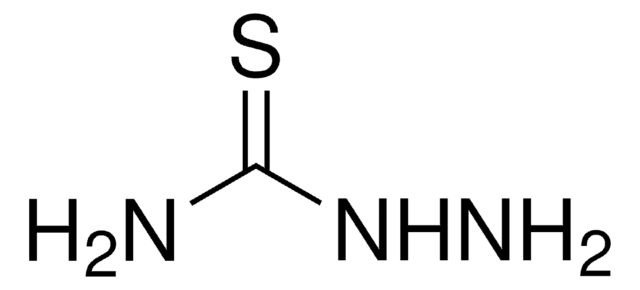
Thiocarbohydrazide
98%
Sinonimo/i:
Thiocarbonyldihydrazide
About This Item
Prodotti consigliati
Saggio
98%
Stato
solid
Punto di fusione
171-174 °C (dec.) (lit.)
Gruppo funzionale
amine
hydrazine
thiourea
Stringa SMILE
NNC(=S)NN
InChI
1S/CH6N4S/c2-4-1(6)5-3/h2-3H2,(H2,4,5,6)
LJTFFORYSFGNCT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Review of transition metal complexes with thiocarbohydrazides: This comprehensive review discusses the coordination chemistry of thiocarbohydrazides with various metals, highlighting their relevance in the synthesis of complex metal compounds used in catalysis and pharmaceutical research (Aly et al., 2023).
- Pd-doped nanocomposites for organometallic catalysis: Thiocarbohydrazide was employed in the fabrication of Pd-doped SBA-15 nanocomposites, applied as catalysts in the synthesis of organometallic compounds, showcasing its utility as a catalyst support material (Kalhor and Dadras, 2021).
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 2 Oral
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.