214930
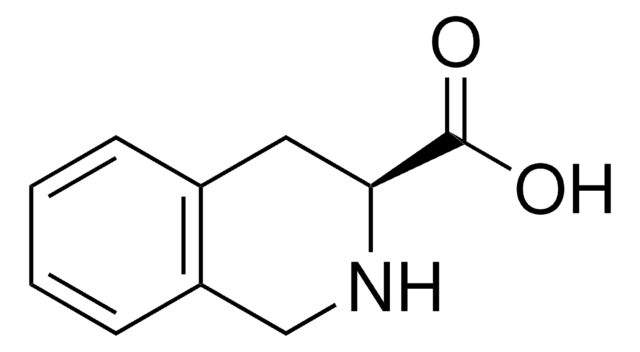
1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid hydrochloride
96%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H11NO2 · HCl
Numero CAS:
Peso molecolare:
213.66
Beilstein:
3723332
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
96%
Punto di fusione
>300 °C (lit.)
Stringa SMILE
Cl[H].OC(=O)C1Cc2ccccc2CN1
InChI
1S/C10H11NO2.ClH/c12-10(13)9-5-7-3-1-2-4-8(7)6-11-9;/h1-4,9,11H,5-6H2,(H,12,13);1H
FXHCFPUEIDRTMR-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid was used in the synthesis of 10,10a-dihydroimidazo-[1,5-b]isoquinoline-1,3(2H,5H)-diones, inhibitor of inflammation, apoprotein B-100 biosynthesis and matrix-degrading metalloprotienase.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Alan R Katritzky et al.
The Journal of organic chemistry, 67(23), 8224-8229 (2002-11-09)
Condensations of chiral diamines 11a-c with benzotriazole and formaldehyde gave benzotriazolyl intermediates 12a-c; similar condensations of alpha-amino-amides 10a-c with benzotriazole and paraformaldehyde gave 14a-c. Subsequent treatment of 12a-c and 14a-c with AlCl(3) led to enantiopure tricyclic 1,2,3,5,10,10a-hexahydroimidazo[1,5-b]isoquinolines 1a-c and 2,3,10,10a-tetrahydroimidazo[1,5-b]isoquinolin-1(5H)-ones
Raman K Bakshi et al.
Bioorganic & medicinal chemistry letters, 15(14), 3430-3433 (2005-06-14)
The discovery of 1-amino-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid analogs as potent human melanocortin-4 selective agonists is described.
B C Wilkes et al.
Biopolymers, 34(9), 1213-1219 (1994-09-01)
A molecular mechanics study (grid search and energy minimization) of the highly delta receptor-selective delta opioid antagonist H-Tyr-Tic-Phe-OH (TIP; Tic: tetrahydroisoquinoline-3-carboxylic acid) resulted in four low energy conformers with energies within 2 kcal/mol of that of the lowest energy structure.
M Manning et al.
Journal of peptide science : an official publication of the European Peptide Society, 1(1), 66-79 (1995-01-01)
We have investigated the effects of mono-substitutions with the conformationally restricted amino acid, 1,2,3,4 tetrahydroisoquinoline-3-carboxylic acid (Tic) at position 3 in arginine vasopressin (AVP), at positions 2, 3 and 7 in potent non-selective cyclic AVP V2/V1a antagonists, in potent and
Yingjie Zhang et al.
Current protein & peptide science, 11(8), 752-758 (2011-01-18)
Tic, short for 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, is a kind of unnatural α-amino acids. Due to its distinct geometrical conformation and biological activity, the structure of Tic, regarded as the surrogate of proline and the rigid analogue of phenylalanine or tyrosine, has
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![1,3-Bis[4-(dimethylamino)phenyl]-2,4-dihydroxycyclobutenediylium dihydroxide, bis(inner salt) Dye content 90 %](/deepweb/assets/sigmaaldrich/product/structures/301/519/500149b3-198c-44cf-b952-7e91f54fc48e/640/500149b3-198c-44cf-b952-7e91f54fc48e.png)
![2,4-Bis[4-(N,N-diphenylamino)-2,6-dihydroxyphenyl]squaraine 98%](/deepweb/assets/sigmaaldrich/product/structures/303/054/d8b9c845-3623-4f5a-8a30-ab6731034171/640/d8b9c845-3623-4f5a-8a30-ab6731034171.png)