180351
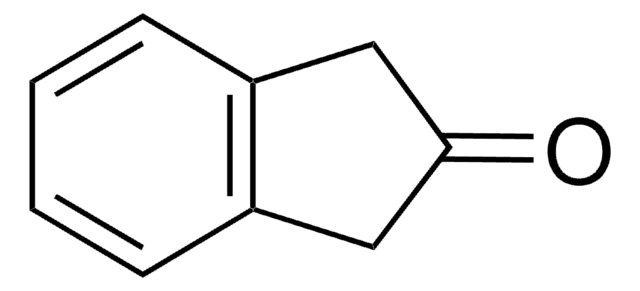
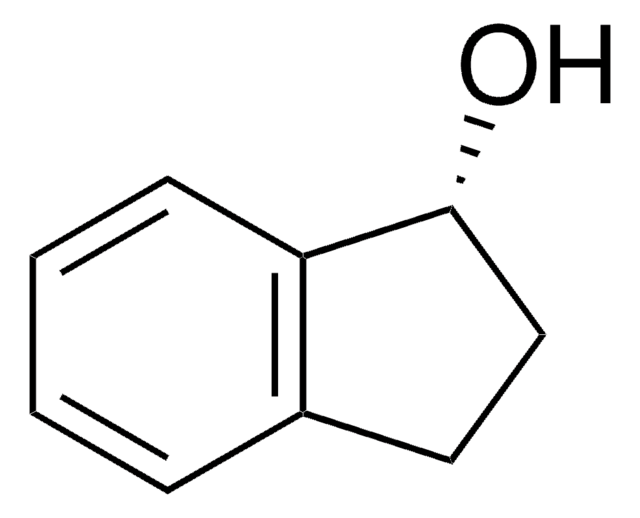
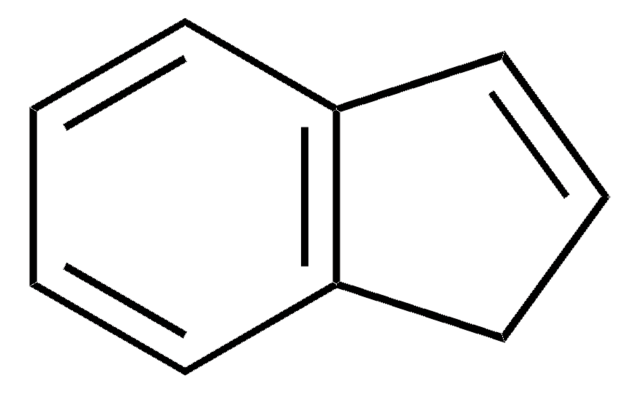
2-Indanol
99%
Sinonimo/i:
2-Hydroxyindan
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H10O
Numero CAS:
Peso molecolare:
134.18
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Stato:
solid
Saggio:
99%
Prodotti consigliati
Saggio
99%
Stato
solid
Punto di fusione
68-71 °C (lit.)
Gruppo funzionale
hydroxyl
Stringa SMILE
OC1Cc2ccccc2C1
InChI
1S/C9H10O/c10-9-5-7-3-1-2-4-8(7)6-9/h1-4,9-10H,5-6H2
KMGCKSAIIHOKCX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2-Indanol is stabilized by internal hydrogen bonding in its most stable form. The resonantly enhanced multiphoton ionization (REMPI) and zero kinetic energy (ZEKE) photoelectron spectroscopy of 2-indanol was studied.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Abdulaziz A Al-Saadi et al.
The journal of physical chemistry. A, 110(44), 12292-12297 (2006-11-03)
2-Indanol in its most stable form is stabilized by internal hydrogen bonding, which exists between the hydroxyl hydrogen atom and the pi-cloud of the benzene ring. A comprehensive ab initio calculation using the MP2/cc-pVTZ level of theory showed that 2-indanol
Yonggang He et al.
The Journal of chemical physics, 124(20), 204306-204306 (2006-06-16)
We report studies of a supersonically cooled 2-indanol using two-color resonantly enhanced multiphoton ionization (REMPI) and two-color zero kinetic energy (ZEKE) photoelectron spectroscopy. In the REMPI experiment, we have identified three conformers of 2-indanol and assigned the vibrational structures of
The Toluene o-Xylene Monooxygenase Enzymatic Activity for the Biosynthesis of Aromatic Antioxidants.
Giuliana Donadio et al.
PloS one, 10(4), e0124427-e0124427 (2015-04-29)
Monocyclic phenols and catechols are important antioxidant compounds for the food and pharmaceutic industries; their production through biotransformation of low-added value starting compounds is of major biotechnological interest. The toluene o-xylene monooxygenase (ToMO) from Pseudomonas sp. OX1 is a bacterial
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.