142719
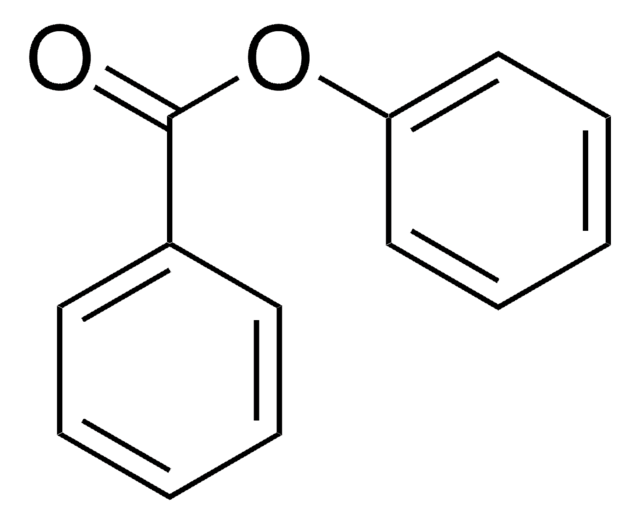
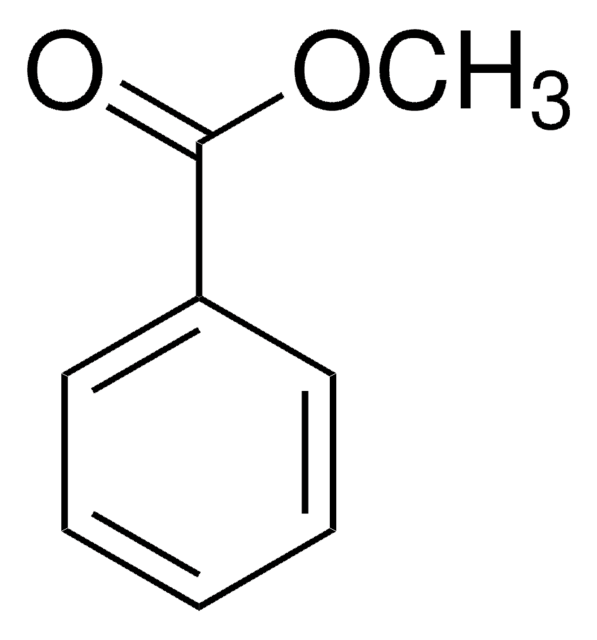
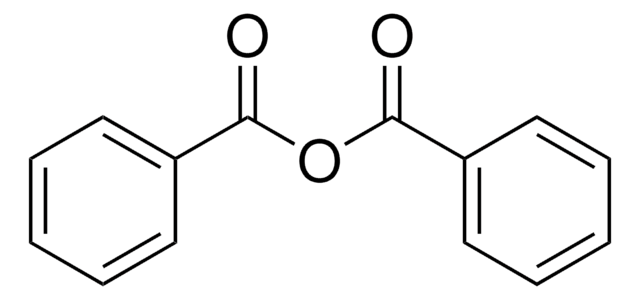
Phenyl benzoate
99%
Sinonimo/i:
Benzoic acid phenyl ester
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99%
Forma fisica
solid
P. eboll.
298-299 °C (lit.)
Punto di fusione
68-70 °C (lit.)
Solubilità
alcohol: freely soluble (hot)
diethyl ether: slightly soluble
water: insoluble
Gruppo funzionale
ester
phenoxy
phenyl
Stringa SMILE
O=C(Oc1ccccc1)c2ccccc2
InChI
1S/C13H10O2/c14-13(11-7-3-1-4-8-11)15-12-9-5-2-6-10-12/h1-10H
FCJSHPDYVMKCHI-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Phenyl benzoate serves as a precursor that undergoes the intramolecular biaryl coupling reaction to produce the intermediate for the synthesis of (−)-steganone.
Applicazioni
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.