117757
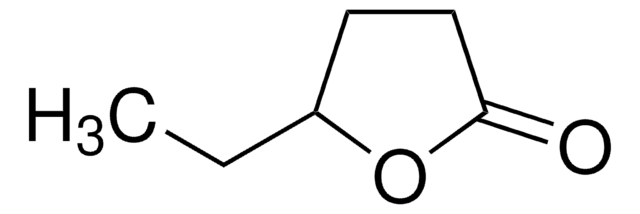
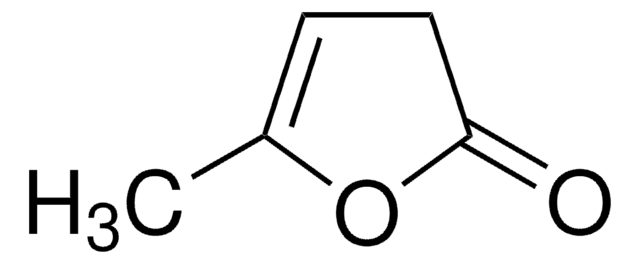
α-Methyl-γ-butyrolactone
98%
Sinonimo/i:
4,5-Dihydro-3-methyl-2(3H)-furanone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H8O2
Numero CAS:
Peso molecolare:
100.12
Beilstein:
80418
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Indice di rifrazione
n20/D 1.432 (lit.)
P. ebollizione
78-81 °C/10 mmHg (lit.)
Solubilità
THF: soluble
Gruppo funzionale
ester
Stringa SMILE
CC1CCOC1=O
InChI
1S/C5H8O2/c1-4-2-3-7-5(4)6/h4H,2-3H2,1H3
QGLBZNZGBLRJGS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
α-Methyl-γ-butyrolactone undergoes benzylation to give racemic α-benzyl-α-methyl-γ-butyrolactone.
Applicazioni
α-Methyl-γ-butyrolactone was used as model compound in Bracketing experiments to investigate the thermodynamically favored site of reaction of pilocarpine.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
163.4 °F - closed cup
Punto d’infiammabilità (°C)
73 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
M Satterfield et al.
Journal of the American Society for Mass Spectrometry, 10(3), 209-216 (1999-03-09)
Analysis of the sites of reaction of a biologically important compound, pilocarpine, a molecule with imidazole and butyrolactone rings connected by a methylene bridge, has been accomplished in a quadrupole ion trap with the aim of characterizing its structure/reactivity relationships.
Eric B Gonzales et al.
The Journal of pharmacology and experimental therapeutics, 309(2), 677-683 (2004-01-27)
Alkyl-substituted butyrolactones have both inhibitory and stimulatory effects on GABA(A) receptors. Lactones with small alkyl substitutions at the alpha-position positively modulate the channel, whereas beta-substituted lactones tend to inhibit the GABA(A) receptor. These compounds mediate inhibition through the picrotoxin site
Hagai Tavori et al.
Bioorganic & medicinal chemistry, 16(15), 7504-7509 (2008-06-24)
Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters and lactones, whereas the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.