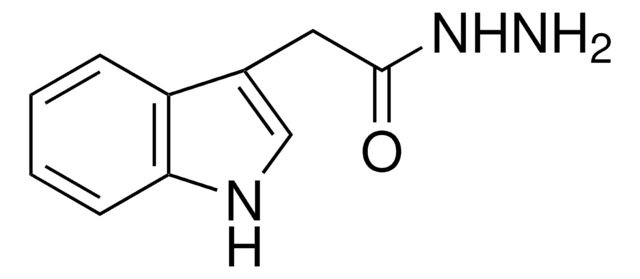
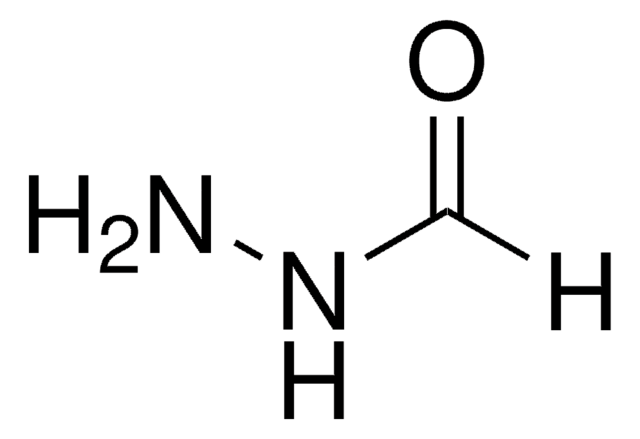
107425
Nicotinic hydrazide
97%
Sinonimo/i:
Nicotinic acid hydrazide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H7N3O
Numero CAS:
Peso molecolare:
137.14
Beilstein:
119299
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Punto di fusione
159-161 °C (lit.)
Solubilità
H2O: soluble 50 mg/mL
Gruppo funzionale
amine
hydrazine
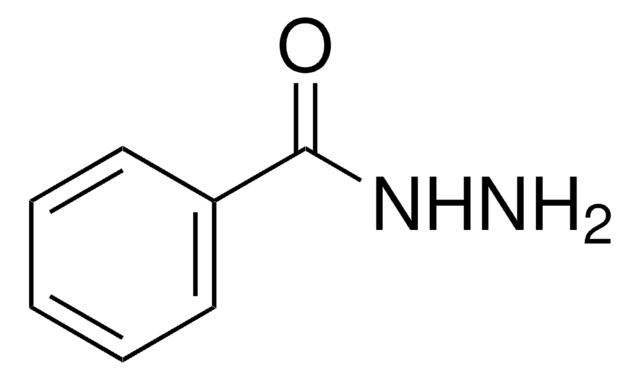
Stringa SMILE
NNC(=O)c1cccnc1
InChI
1S/C6H7N3O/c7-9-6(10)5-2-1-3-8-4-5/h1-4H,7H2,(H,9,10)
KFUSANSHCADHNJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Nicotinic hydrazide is a heterocyclic compound that can be used to synthesize Schiff bases.
Applicazioni
Nicotinic hydrazide was used in hydrazone library formation. It was used to study the oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase.
Azioni biochim/fisiol
Nicotinic hydrazide is an inhibitor of peroxidase enzyme. It forms solid metal complexes having strong biological activity.
Nota sulla preparazione
Nicotinic hydrazide dissolves in water at a concentration of 50 mg/ml to form a clear, colourless solution.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
V Goral et al.
Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1347-1352 (2001-02-15)
Dynamic combinatorial libraries are mixtures of compounds that exist in a dynamic equilibrium and can be driven to compositional self adaptation via selective binding of a specific assembly of certain components to a molecular target. We present here an extension
Thermo-chemical behavior of solid nicotinic hydrazide metal complexes in correlation with their stoichiometry.
Sekkina MM and El-Azm MG.
Thermochimica Acta, 77(1), 211-218 (1984)
H A Shoeb et al.
Antimicrobial agents and chemotherapy, 27(3), 399-403 (1985-03-01)
Oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase at the expense of H2O2 yielded reactive species which were able to reduce nitroblue tetrazolium and bleach p-nitrosodimethylaniline. Nicotinic acid hydrazide oxidation did not cause these effects. At slightly alkaline pH
Alireza Moradi et al.
Archiv der Pharmazie, 343(9), 509-518 (2010-09-02)
A series of 2-phenoxynicotinic acid hydrazides were synthesized and evaluated for their analgesic and anti-inflammatory activities. Several compounds having an unsubstituted phenyl/4-pyridyl or C-4 methoxy substituent on the terminal phenyl ring showed moderate to high analgesic or anti-inflammatory activity in
[Tautomerism of 2-hydrazino-4-phenylthiazole<-->4-phenylthiazol-2-one hydrazone. Derivatives of acids. II. (4-phenyl-3-R-thiazol-2-ylidene) and beta-methyl-beta-(4-phenylthiazol-2-yl) hydrazides of picolinic, nicotinic, and isonicotinic acid].
L Bielak et al.
Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina, 44, 41-51 (1989-01-01)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.