All Photos(1)
About This Item
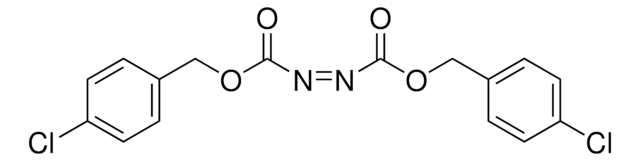
Linear Formula:
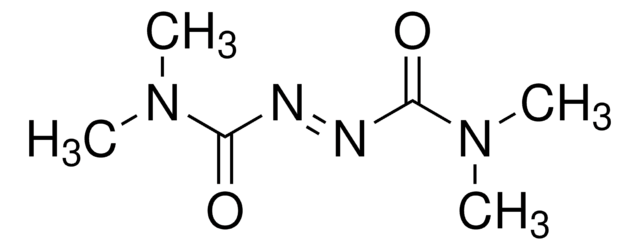
C6H5CH2OCON=NCOOCH2C6H5
CAS Number:
Molecular Weight:
298.29
Beilstein:
2298734
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
solid
mp
43-47 °C (lit.)
functional group
azo
phenyl
storage temp.
2-8°C
SMILES string
O=C(OCc1ccccc1)\N=N\C(=O)OCc2ccccc2
InChI
1S/C16H14N2O4/c19-15(21-11-13-7-3-1-4-8-13)17-18-16(20)22-12-14-9-5-2-6-10-14/h1-10H,11-12H2/b18-17+
InChI key
IRJKSAIGIYODAN-ISLYRVAYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dibenzyl azodicarboxylate undergoes[4+2] cycloaddition reaction with glycal to yield 2-aminoglycosides.
Application
Dibenzyl azodicarboxylate was used as electrophilic reagent in the synthesis of C-glycosyl α-amino acids via proline-catalyzed α-amination of C-glycosylalkyl aldehydes. It was used in enantioselective synthesis of optically active pyrazolidine derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[4+ 2] Cycloaddition reaction of dibenzyl azodicarboxylate and glycals.
Leblanc Y, et al.
Journal of the American Chemical Society, 111(8), 2995-3000 (1989)
Shengming Ma et al.
Organic letters, 6(13), 2193-2196 (2004-06-18)
[reaction: see text] Optically active pyrazolidine derivatives have been constructed by the Cu- and Pd-catalyzed asymmetric one-pot tandem addition-cyclization reaction of 2-(2',3'-dienyl)-beta-ketoesters, organic halides, and dibenzyl azodicarboxylate. The absolute configurations of the final products depend on the structure of the
Andrea Nuzzi et al.
Organic letters, 10(20), 4485-4488 (2008-09-16)
Non-natural axially and equatorially linked C-glycosyl alpha-amino acids (glycines, alanines, and CH2-serine isosteres) with either S or R alpha-configuration were prepared by D- and L-proline-catalyzed (de >95%) alpha-amination of C-glycosylalkyl aldehydes using dibenzyl azodicarboxylate as the electrophilic reagent.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service