255920
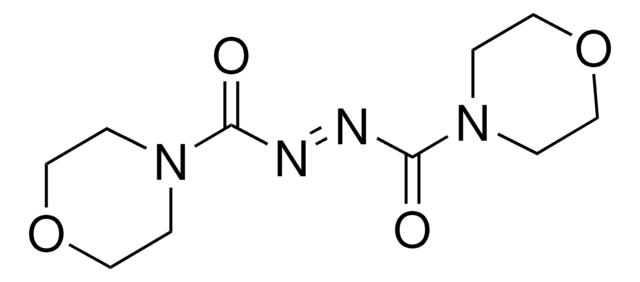
1,1′-(Azodicarbonyl)dipiperidine
99%
Synonym(s):
1,1′-Azobis(N,N-pentamethyleneformamide), ADD, Azodicarboxylic acid dipiperidide, NSC 356027, SR 4077
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H20N4O2
CAS Number:
Molecular Weight:
252.31
Beilstein:
261917
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
134-136 °C (lit.)
functional group
azo
SMILES string
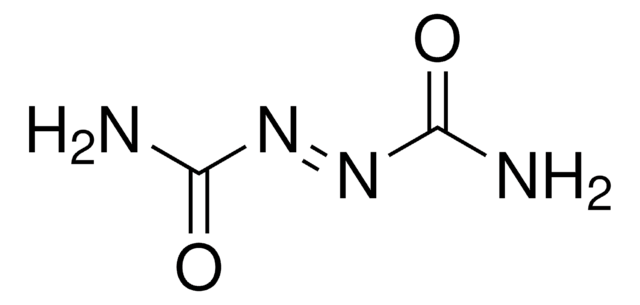
O=C(\N=N\C(=O)N1CCCCC1)N2CCCCC2
InChI
1S/C12H20N4O2/c17-11(15-7-3-1-4-8-15)13-14-12(18)16-9-5-2-6-10-16/h1-10H2/b14-13+
InChI key
OQJBFFCUFALWQL-BUHFOSPRSA-N
Application
Reactant for preparation of:
Reactant for:
- Polyfluoroalkylated tripyrazolylmethane ligands
- (-)-Hygromycin A via Mitsunobu glycosylation
- Optically active α,α-disubstituted amino acids via Mitsunobu reaction
- Aza-β-lactams through [2+2] cycloaddition reactions
- Glycosyl disulfides
- Pyridine ether PPAR agonists
- S-glycosyl amino acid building blocks for combinatorial neoglycopeptide synthesis
- Histamine H3 receptor antagonists
Reactant for:
- Mitsunobu inversion reactions
Used in a study of the copper-catalyzed addition of aryboronic acids to azodicarboxyl derivatives providing aryl-substituted hydrazides.
Widely used reagent for the Mitsunobu reaction
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hiroki Sato et al.
Bioorganic & medicinal chemistry, 10(5), 1595-1610 (2002-03-12)
Structure--activity relationship studies of 1beta-methyl-2-[(3S,5R)-5-(4-aminomethylphenyl)pyrrolidin-3-ylthio]carbapenems, especially those pertaining to the relationship between antibacterial activity and side-chain structure were conducted. These studies suggested that the trans-(3S,5R)-5-phenylpyrrolidin-3-ylthio side-chain and the aminomethyl group at the 4-position of the phenyl ring play a key
E A Bump et al.
International journal of radiation oncology, biology, physics, 12(8), 1533-1535 (1986-08-01)
Several analogs of the glutathione (GSH) oxidizing reagent diamide [diazenedicarboxylic acid bis(N,N'-diethylamide)] were tested as radiosensitizers of aerobic cells. In general, radiosensitization correlates with the rate of reaction with cellular reducing agents and occurs only when the reductive capacity of
Tetrahedron Letters, 34, 1639-1639 (1993)
M A Baker et al.
Radiation research, 113(2), 346-355 (1988-02-01)
The mechanism of radiosensitization by diazenedicarboxylic acid bis(N),N-piperidide (SR 4077), a less toxic analog of diamide, was studied using Chinese hamster ovary cells. SR 4077 gave an average SER of 1.58 for postirradiation incubations of 0.5, 1.0, or 2.0 h.
E A Bump et al.
International journal of radiation oncology, biology, physics, 29(2), 249-253 (1994-05-15)
To determine whether biological effects of radiation, such as apoptosis, that differ from classical clonogenic cell killing, can be modified with agents that would not be expected to modify classical clonogenic cell killing. This would expand the range of potential
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service