All Photos(1)
About This Item
Empirical Formula (Hill Notation):
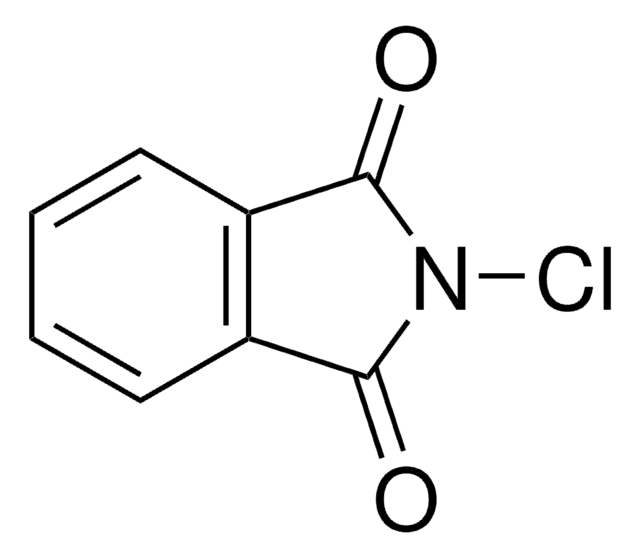
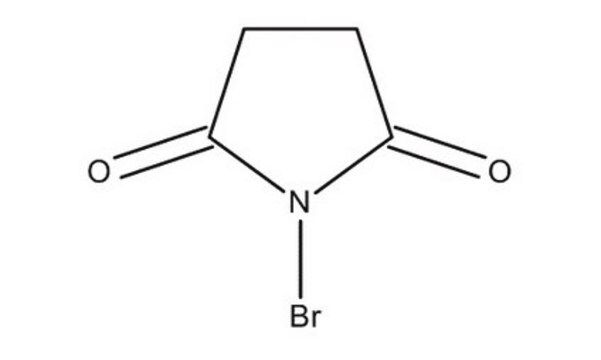
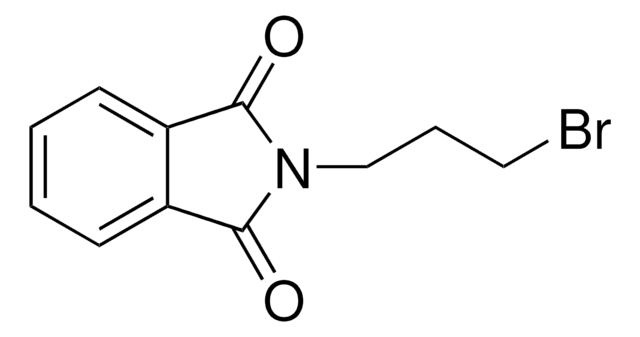
C8H4BrNO2
CAS Number:
Molecular Weight:
226.03
Beilstein:
131211
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
194-198 °C (lit.)
functional group
imide
SMILES string
BrN1C(=O)c2ccccc2C1=O
InChI
1S/C8H4BrNO2/c9-10-7(11)5-3-1-2-4-6(5)8(10)12/h1-4H
InChI key
MARXMDRWROUXMD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N-Bromophthalimide has been used:
- as reagent in allylic amination reactions of alkenes
- brominating reagent in enantioselective synthesis of multisubstituted biaryl derivatives by chiral phosphoric acid catalyzed asymmetric bromination
- as a titrant in titrimetric determination of isoniazid in pure form or in tablets
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A M el-Brashy et al.
Journal of pharmaceutical and biomedical analysis, 10(6), 421-426 (1992-06-01)
Two methods are proposed for the determination of isoniazid in pure form or in tablets. In the first method chlorpromazine hydrochloride, when treated with 2-iodoxybenzoic acid as an oxidant in 50% w/v o-phosphoric acid solution, is oxidized to chlorpromazine free
Titrimetric methods for the determination of some sulpha drugs using N-bromophthalimide and N-bromosaccharin.
K G Kumar et al.
The Analyst, 113(9), 1369-1372 (1988-09-01)
Tejas P Pathak et al.
Journal of the American Chemical Society, 134(14), 6120-6123 (2012-04-03)
We report the site-selective bromination of vancomycin to produce, with substantial efficiency, previously unknown monobromovancomycins, a dibromovancomycin, and a tribromovancomycin. We document the inherent reactivity of native vancomycin toward N-bromophthalimide. We then demonstrate significant rate acceleration and perturbation of the
Feng Chen et al.
Journal of the American Chemical Society, 135(4), 1232-1235 (2013-01-15)
A catalytic asymmetric bromocyclization of trisubstituted olefinic amides that uses a C(2)-symmetric mannitol-derived cyclic selenium catalyst and a stoichiometric amount of N-bromophthalimide is reported. The resulting enantioenriched pyrrolidine products, which contain two stereogenic centers, can undergo rearrangement to yield 2,3-disubstituted
Titrimetric determination of para-aminobenzoic acid using N-bromophthalimide and N-bromosaccharin.
K G Kumar et al.
Journal of pharmaceutical and biomedical analysis, 7(5), 627-631 (1989-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service