D125806
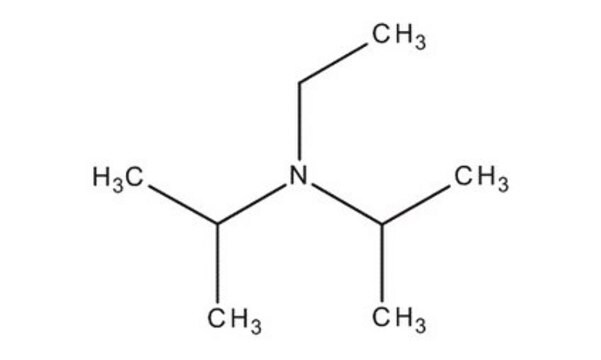
N,N-Diisopropylethylamine
ReagentPlus®, ≥99%
Synonym(s):
N-Ethyldiisopropylamine, DIPEA, Ethyldiisopropylamine, ‘Hünig’s base’
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(4)
Select a Size
Change View
About This Item
Linear Formula:
[(CH3)2CH]2NC2H5
CAS Number:
Molecular Weight:
129.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor pressure
31 mmHg ( 37.7 °C)
Quality Level
product line
ReagentPlus®
Assay
≥99%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
127 °C (lit.)
mp
<−50 °C (lit.)
density
0.742 g/mL at 25 °C (lit.)
SMILES string
CCN(C(C)C)C(C)C
Looking for similar products? Visit Product Comparison Guide
General description
N,N-Diisopropylethylamine, also known as Hünig′s base, is a sterically hindered amine. It is a non-nucleophilic base commonly employed for substitution reactions. It acts as an activator for chiral iridium N, P ligand complexes, which can be utilized in the hydrogenation of α, β-unsaturated nitriles. The influence of varying concentration of N,N-diisopropylethylamine on the synthesis of olvanil in the presence of lipase catalyst has been investigated.
Application
N,N-Diisopropylethylamine may be used in the synthesis of mannosylated ovalbumin peptides.
Proton scavenger used in peptide coupling, enolboration, Pd(0)-catalyzed alkoxycarbonylation of allyl phosphates and acetates, and as a catalyst in vinyl sulfone synthesis.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.1 °F
Flash Point(C)
9.5 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic Communications, 23, 3073-3073 (1993)
The Journal of Organic Chemistry, 58, 7162-7162 (1993)
The Journal of Organic Chemistry, 58, 1538-1538 (1993)
The Journal of Organic Chemistry, 59, 695-695 (1994)
Lipase-catalysed synthesis of olvanil in organic solvents.
Reyes-Duarte D, et al.
Biotechnology Letters, 24(24), 2057-2061 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service