441791
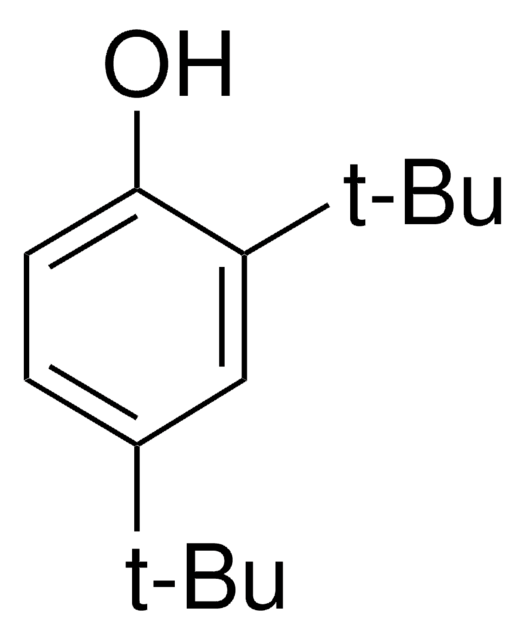
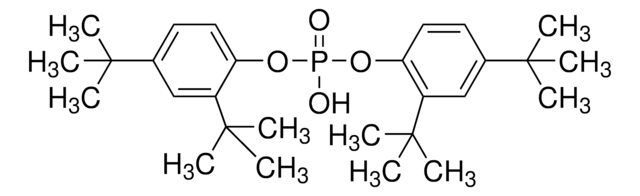
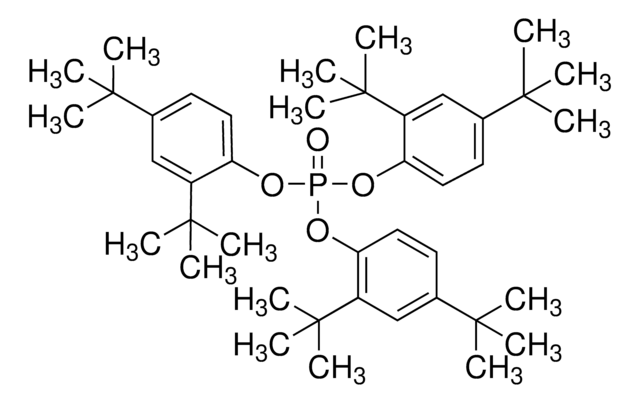
Tris(2,4-di-tert-butylphenyl) phosphite
Synonym(s):
2,4-Bis(1,1-dimethylethyl)phenol phosphite (3:1), Tri(2,4-di-tert -butylphenyl) phosphite, Tri(2,4-di-t -butylphenyl) phosphite
About This Item
Recommended Products
Quality Level
mp
181-184 °C (lit.)
SMILES string
CC(C)(C)c1ccc(OP(Oc2ccc(cc2C(C)(C)C)C(C)(C)C)Oc3ccc(cc3C(C)(C)C)C(C)(C)C)c(c1)C(C)(C)C
InChI
1S/C42H63O3P/c1-37(2,3)28-19-22-34(31(25-28)40(10,11)12)43-46(44-35-23-20-29(38(4,5)6)26-32(35)41(13,14)15)45-36-24-21-30(39(7,8)9)27-33(36)42(16,17)18/h19-27H,1-18H3
InChI key
JKIJEFPNVSHHEI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Phenolic Antioxidants are added to polyers for controlling their degradation process. Irgafos 168, Irganox 1010, Ethanox 330, Irganox 1076, BHT, and Irgafos 168 are often used as antioxidants to prevent the degradation of polypropylene polymer formulations. This HPLC method is for the quantitation of an additive or mix of additives in a polymeric material.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![3,9-Bis(octadecyloxy)-2,4,8,10-tetraoxa-3,9-diphosphaspiro[5.5]undecane](/deepweb/assets/sigmaaldrich/product/structures/426/453/2ce260eb-38be-4a9d-b432-9710a0c9a290/640/2ce260eb-38be-4a9d-b432-9710a0c9a290.png)