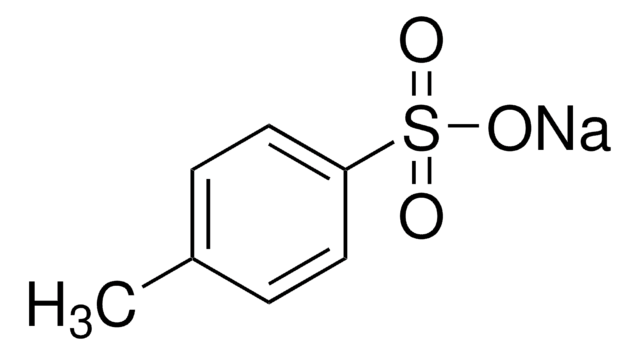
293768
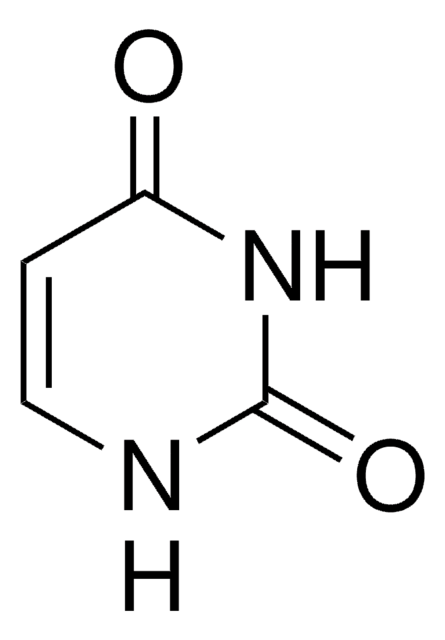
1-Methyluracil
99%
Synonym(s):
1-Methyl-2,4(1H,3H)-pyrimidinedione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6N2O2
CAS Number:
Molecular Weight:
126.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
236-238 °C (lit.)
solubility
1 M NaOH: soluble 50 mg/mL, clear, colorless
SMILES string
CN1C=CC(=O)NC1=O
InChI
1S/C5H6N2O2/c1-7-3-2-4(8)6-5(7)9/h2-3H,1H3,(H,6,8,9)
InChI key
XBCXJKGHPABGSD-UHFFFAOYSA-N
General description
1-Methyluracil is of special importance in biochemistry, since uracil attaches ribose in ribonucleic acid (RNA) just precisely at the N1 atom. H-bond complex formation between 1-methyluracil and glycine has been investigated by theoretical calculations and FT-IR spectroscopy in Ar matrices. It forms 1:1 complexes with 9-ethyl-8-bromo-2,6-diaminopurine and the complex structure has been determined by three-dimensional X-ray diffraction methods.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Marek Boczar et al.
The Journal of chemical physics, 128(16), 164506-164506 (2008-05-02)
Theoretical simulation of the band shape and fine structure of the N-H(D) stretching band is presented for 1-methyluracil and its deuterated derivative taking into account anharmonic coupling between the high-frequency N-H(D) stretching and the low-frequency N...O stretching vibrations, resonance interaction
L F Sukhodub et al.
Biofizika, 34(2), 181-186 (1989-03-01)
The hydration of nucleotide bases of m9Ade(A), m1Ura(U) and a complementary pair A.U was studied by field ionization mass-spectrometry at room and low (170 K) temperatures in vacuum. Enthalpies of A.U-pair formation and its monohydrate A.U(H2O) were measured using temperature
W T Klooster et al.
Acta crystallographica. Section B, Structural science, 48 ( Pt 2), 217-227 (1992-04-01)
1-Methyluracil (1-methyl-2,4-dioxopyrimidine), C5H6-N2O2, M(r) = 126.12, orthorhombic, Ibam, a = 13.188 (6), b = 13.175 (5), c = 6.214 (3) A, V = 1079.7 (8) A3, Z = 8, Dx = 1.552 g cm-3, lambda (Mo K alpha) = 0.7107
A Leś et al.
Journal of biomolecular structure & dynamics, 15(4), 703-715 (1998-03-26)
Theoretical quantum mechanical ab initio Hartree-Fock calculations on molecular systems, modeling processes related to the specificity of thymidylate synthase inactivation are reported. We considered several steps of the methylation of the substrate dUMP and 4- or 5-mono- and 4,5-bisubstituted dUMP
Natalja Vogt et al.
The journal of physical chemistry. A, 117(44), 11374-11381 (2013-10-31)
As far as fundamental knowledge is concerned, the methyl derivatives of uracil can be considered as the simplest objects for studying the structural effects due to the substitution in the pyrimidyne nucleobases. From this point of view, 1-methyluracil is of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service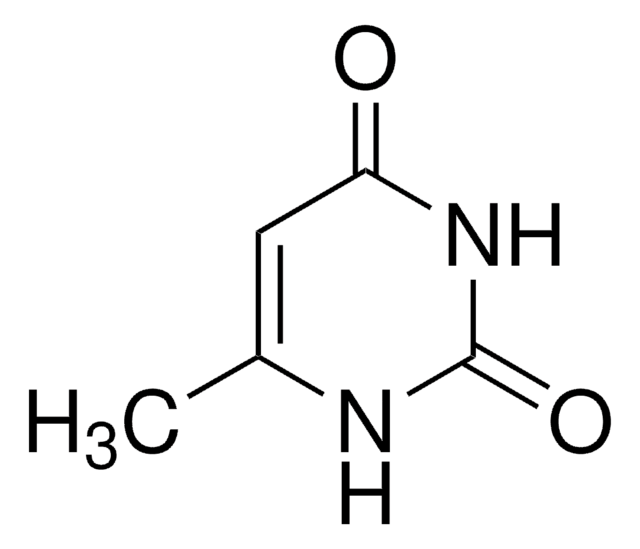


![6-Methylthieno[2,3-d]pyrimidine-2,4(1H,3H)-dione](/deepweb/assets/sigmaaldrich/product/structures/393/943/c932f315-dd4b-4939-aea6-646238005e48/640/c932f315-dd4b-4939-aea6-646238005e48.png)