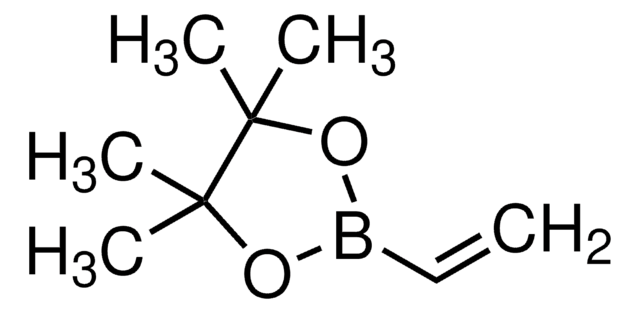
731528
trans-2-Ethoxyvinylboronic acid pinacol ester
95%
Synonym(s):
(E)-2-(2-Ethoxyvinyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, (E)-2-Ethoxyvinylboronic acid pinacol ester
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C10H19BO3
CAS Number:
Molecular Weight:
198.07
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.447
density
0.935 g/mL at 25 °C
functional group
ether
storage temp.
2-8°C
SMILES string
CCO\C=C\B1OC(C)(C)C(C)(C)O1
InChI
1S/C10H19BO3/c1-6-12-8-7-11-13-9(2,3)10(4,5)14-11/h7-8H,6H2,1-5H3/b8-7+
InChI key
MRAYNLYCQPAZJN-BQYQJAHWSA-N
Related Categories
Application
trans-2-Ethoxyvinylboronic acid pinacol ester is a boronic ester commonly used in Suzuki-Miyaura cross-coupling.
This reaction is a key step to synthesize:
This reaction is a key step to synthesize:
- Azaindole and diazaindoles from chloroamino-N-heterocycles.
- Doryanine and its derivatives from 2-bromobenzoic acid.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
188.1 °F
Flash Point(C)
86.7 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Structure-and reactivity-based development of covalent inhibitors of the activating and gatekeeper mutant forms of the epidermal growth factor receptor (EGFR).
Ward, Richard A et al.
Journal of Medicinal Chemistry, 56(17), 7025-7048 (2013)
Total Synthesis of the Illicium-Derived Sesquineolignan Simonsol C.
Nugent, Jeremy et al.
Organic Letters, 18(15), 3798-3801 (2016)
Two-step synthesis of aza-and diazaindoles from chloroamino-N-heterocycles using ethoxyvinylborolane.
Whelligan, Daniel K et al.
The Journal of Organic Chemistry, 75(1), 11-15 (2009)
Efficient and rapid synthesis of N-substituted isoquinolin-1-ones under mild conditions: Facile access to doryanine derivatives
Takwale AD, et al.
Tetrahedron Letters, 60(18), 1259-1261 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)