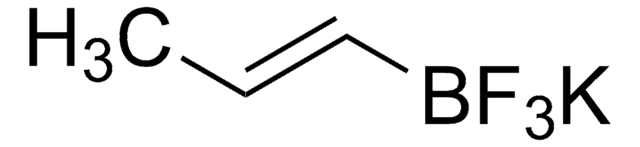
655228
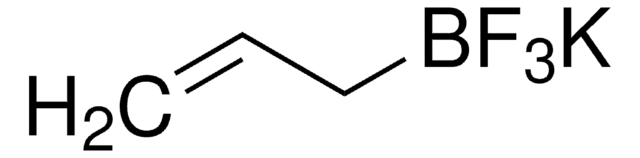
Potassium vinyltrifluoroborate
95%
Synonym(s):
Potassium (ethenyl)trifluoroborate
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
SMILES string
[K+].F[B-](F)(F)C=C
InChI
1S/C2H3BF3.K/c1-2-3(4,5)6;/h2H,1H2;/q-1;+1
InChI key
ZCUMGICZWDOJEM-UHFFFAOYSA-N
General description
Potassium vinyltrifluoroborate is an air- and water-stable potassium organotrifluoroborate that can be utilized in coupling reactions under relatively mild conditions.
Application
- Suzuki Miyaura cross-coupling reactions and polymerization reactions
- Synthesis of photonic crystals
- Synthesis of sensitizers for dye-sensitized solar cells
- Mannich / diastereoselective hydroamination reaction sequence
Organotrifluoroborates as versatile and stable boronic acid surrogates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Potassium trifluoroborates are a special class of organoboron reagents that offer several advantages over the corresponding boronic acids and esters in that they are moisture- and air-stable, and are remarkably compliant with strong oxidative conditions.
These bench stable Potassium Organotrifluoroborates are useful for Suzuki-Miyaura cross-coupling reactions and have also been used for a variety of other C-C bond forming reactions. Importantly, these reagents are compatible with a wide range of functional groups and are stable to many commonly used and harsh reaction conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)