637998
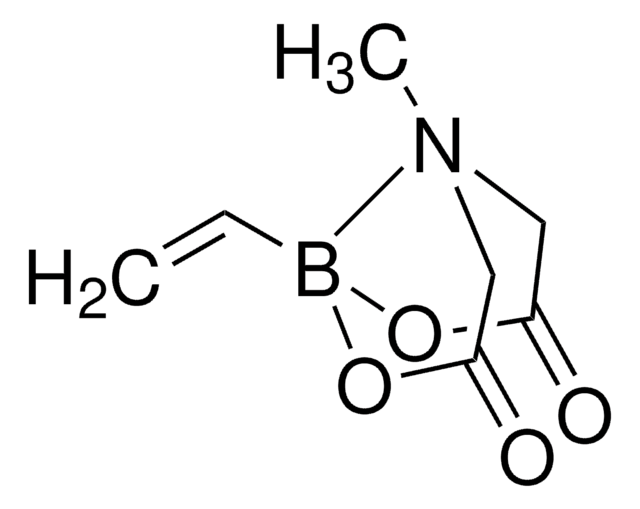
Vinylboronic anhydride pyridine complex
95%
Synonym(s):
2,4,6-Trivinylcyclotriboroxane pyridine complex, Trivinyl-boroxin pyridine complex, Trivinylcyclotriboroxane pyridine complex
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H9B3O3 · C5H5N
CAS Number:
Molecular Weight:
240.67
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
storage temp.
−20°C
SMILES string
c1ccncc1.C=Cb2ob(C=C)ob(C=C)o2
InChI
1S/C6H9B3O3.C5H5N/c1-4-7-10-8(5-2)12-9(6-3)11-7;1-2-4-6-5-3-1/h4-6H,1-3H2;1-5H
InChI key
YLHJACXHRQQNQR-UHFFFAOYSA-N
Application
Reagent used for
Reagent used for Preparation of
- Suzuki-Miyaura cross-coupling
- Stereoselective synthesis via Palladium-catalyzed carboamination
- Alkyl-connected 2-amino-6-vinylpurine (AVP) crosslinking agent to cytosine base in RNA
- Kaiser oxime resin-derived palladacycle as a recoverable polymeric precatalyst in Suzuki-Miyaura cross-coupling reactions in aqueous media
- Kinetic resolution of phosphoryl and sulfonyl esters of binaphthol derivatives via Pd-catalyzed alcoholysis of their vinyl ethers
- Stereoselective isomerization of N-allyl aziridines into Z-enamines by using rhodium hydride catalysis
- Kinetic resolution of axially chiral biaryl derivatives via palladium/chiral diamine ligand-catalyzed alcoholysis
- Transition metal-catalyzed alkenylation of aziridines, cycloaddition and thermal rearrangement reactions
- Intramolecular Heck reaction strategy for synthesis of functionalized tetrahydroanthracenes
Reagent used for Preparation of
- BACE-1 inhibitors and SAR of cyclic sulfone hydroxyethylamines
- Distorted spiropentanes
- Small molecule bradykinin B2 receptor antagonists in angioedema therapy
- Enol Ethers
- Styryl cyclobutanone
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
176.0 °F - closed cup
Flash Point(C)
80 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kuan-Jen Su et al.
The Journal of organic chemistry, 75(21), 7494-7497 (2010-10-14)
Tetravinylbenzene 4 was prepared in nearly quantitative yield from commercially available tetrabromobenzene; the improved, one-step procedure now employs Suzuki-Miyaura cross-coupling conditions. Intermolecular cyclopropanation of 4 with dibromocarbene gave a series of gem-dibromide adducts. Intramolecular cyclopropanation of monoadduct 5, putatively by
Masahiro Murakami et al.
Organic letters, 7(10), 2059-2061 (2005-05-07)
Two structurally distinct carbocycles were selectively obtained by the reactions of 2-(o-styryl)cyclobutanones promoted by ytterbium salts. Treatment of the cyclobutanones with YbCl(3) in 1,4-dioxane at 100 degrees C afforded 2-(2-chloroethyl)naphthalenes. On the other hand, the reaction with Yb(OTf)(3) in chlorobenzene
Product subclass 3: enol ethers
Milata, V.; Radl, S.; Voltrova, S.
Sci. Synth., 32, 589-756 (2008)
Derek S Tsang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(3), 886-894 (2007-11-10)
In the presence of rhodium(I) hydride catalysts, tertiary N-allylamines are known to isomerise into E enamines. In contrast, we have recently found that N-allylaziridines isomerise to form Z enamines. On the basis of literature data, the most likely mechanism of
Kinetic resolution of phosphoryl and sulfonyl esters of 1,1'-bi-2-naphthol via Pd-catalyzed alcoholysis of their vinyl ethers
Sakuma, T.; et al.
Tetrahedron, 19, 1593-1599 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service