所有图片(1)
About This Item
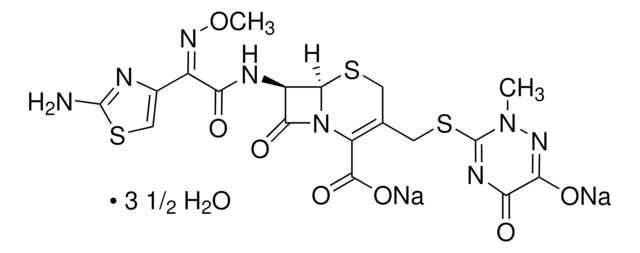
经验公式(希尔记法):
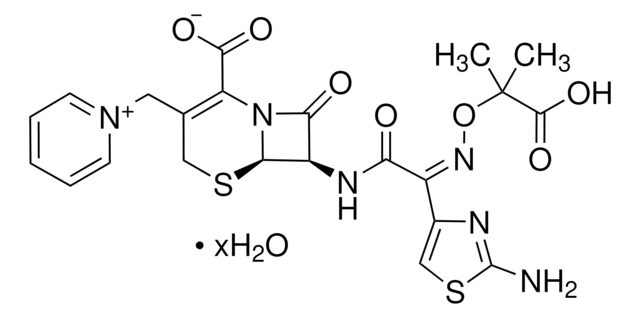
C22H22N6O7S2 · 5H2O
CAS号:
分子量:
636.65
MDL號碼:
分類程式碼代碼:
41116107
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
ceftazidime
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
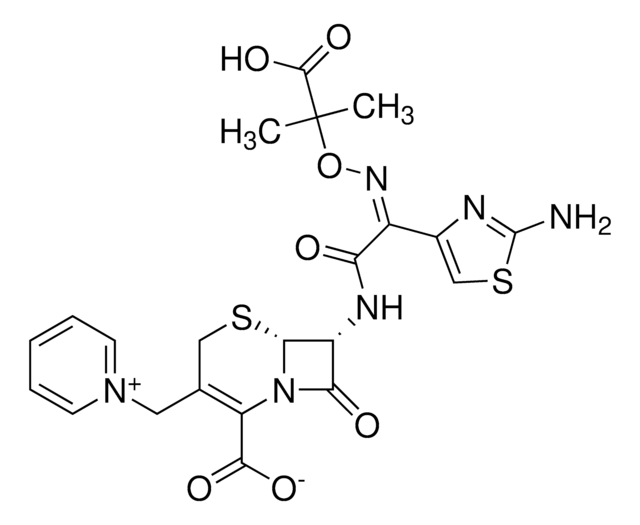
SMILES 字串
[s]1c(nc(c1)\C(=N\OC(C)(C)C(=O)[O-])\C(=O)N[C@H]2[C@H]3SCC(=C(N3C2=O)C(=O)[O-])C[n+]4ccccc4)N.O.O.O.O.O.[H+]
InChI
1S/C22H22N6O7S2.5H2O/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27;;;;;/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34);5*1H2/b26-13-;;;;;/t14-,18-;;;;;/m1...../s1
InChI 密鑰
NMVPEQXCMGEDNH-TZVUEUGBSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ceftazidime pentahydrate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Ceftazidime
- Ceftazidime Injection
- Ceftazidime for Injection
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Kevin M Pegg et al.
Antimicrobial agents and chemotherapy, 57(10), 5122-5126 (2013-07-10)
IMP-type enzymes constitute a clinically important family of metallo-β-lactamases that has grown dramatically in the past decade to its current 45 known members. Here, we report the biochemical characterization of IMP-30 in comparison to IMP-1, from which it deviates by
A Adu et al.
Drugs, 50(3), 423-439 (1995-09-01)
Six parenteral third generation cephalosporins have been introduced into clinical use in the past 10 years. The 3 most frequently available agents are cefotaxime, ceftriaxone and ceftazidime. These 3 third generation cephalosporins are characterised by a broad spectrum of activity
Dandan He et al.
Antimicrobial agents and chemotherapy, 57(8), 4068-4071 (2013-06-12)
The chimeric bla(CTX-M-123) gene was identified in two ceftazidime-resistant Escherichia coli isolates from animals in different Chinese provinces. Like other CTX-M-1/9 group hybrids (CTX-M-64 and CTX-M-132), the ends (amino acids 1 to 135 and 234 to 291) of CTX-M-123 match
C J Helm et al.
Ophthalmology, 104(5), 838-843 (1997-05-01)
Pseudomonal scleritis is a serious and potentially blinding infection that usually is resistant to medical management. Results for three patients with pseudomonal scleritis who were treated with both topical anti-infectives and a combination of intravenous ceftazidime and aminoglycoside are presented
L Knapp et al.
Journal of applied microbiology, 115(5), 1117-1126 (2013-08-06)
The extensive use of microbicides in a wide range of applications has been questioned with regard to their role in the development of bacterial resistance to antimicrobials. This study aims to measure the phenotypic and genotypic changes in Burkholderia lata
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门