推荐产品
等級
certified reference material
TraceCERT®
品質等級
產品線
TraceCERT®
CofA
current certificate can be downloaded
包裝
ampule of 1 mL
濃度
2000 μg/mL in dichloromethane
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
cleaning products
cosmetics
environmental
food and beverages
personal care
形式
single component solution
儲存溫度
2-30°C
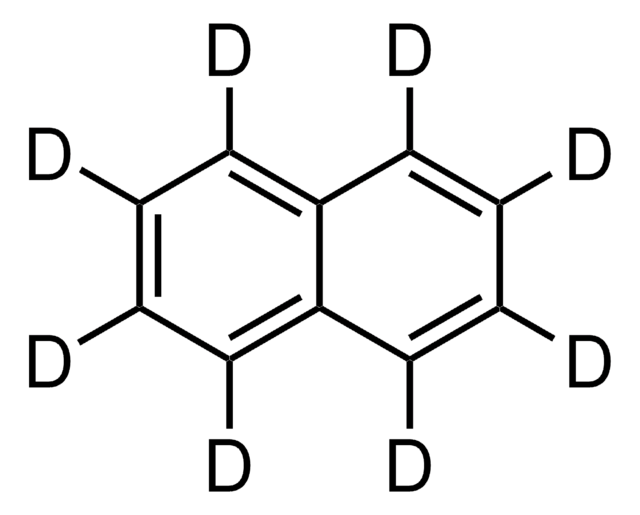
SMILES 字串
c21c3c4c(c2ccc5c1cccc5)cccc4ccc3
InChI
1S/C20H12/c1-2-8-15-13(5-1)11-12-17-16-9-3-6-14-7-4-10-18(19(14)16)20(15)17/h1-12H
InChI 密鑰
KHNYNFUTFKJLDD-UHFFFAOYSA-N
一般說明
應用
其他說明
法律資訊
訊號詞
Danger
危險分類
Aquatic Chronic 3 - Carc. 1B - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Central nervous system
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
实验方案
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持![苯并[k]荧蒽 for fluorescence, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/277/320/3e615f9f-3887-40f6-b176-bc1eb9b4832c/640/3e615f9f-3887-40f6-b176-bc1eb9b4832c.png)
![苯并[e]芘 98%](/deepweb/assets/sigmaaldrich/product/structures/162/859/cd1f8e1f-2539-4f36-be04-8bad9d301215/640/cd1f8e1f-2539-4f36-be04-8bad9d301215.png)
![苯并[b]荧蒽 98%](/deepweb/assets/sigmaaldrich/product/structures/175/744/6fa5fca2-b6ec-47b6-ab7a-fe895843f226/640/6fa5fca2-b6ec-47b6-ab7a-fe895843f226.png)



![苯并[a]芘 ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)


![茚并[1,2,3-cd]比 analytical standard](/deepweb/assets/sigmaaldrich/product/structures/231/153/b0b230c2-efa0-4f43-a261-66b931ead3d2/640/b0b230c2-efa0-4f43-a261-66b931ead3d2.png)