推荐产品
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
protect from light
顏色
yellow
溶解度
DMSO: >5 mg/mL
儲存溫度
room temp
SMILES 字串
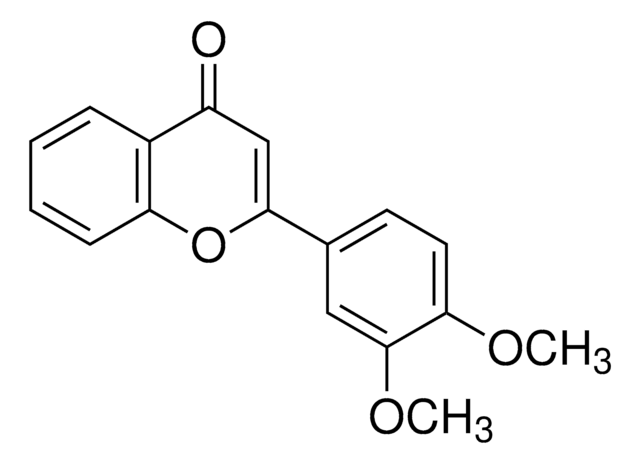
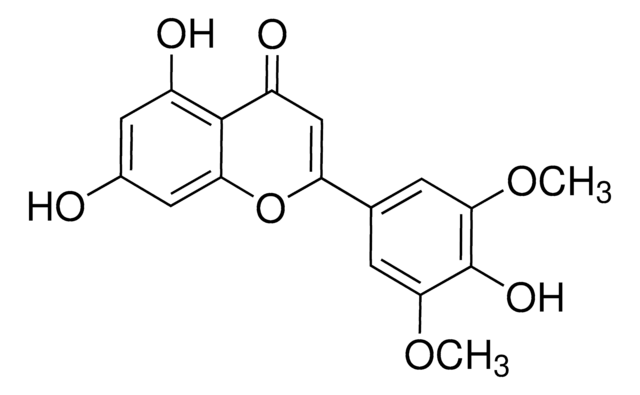
COc1ccc(c(OC)c1)C2=CC(=O)c3cc(OC)ccc3O2
InChI
1S/C18H16O5/c1-20-11-5-7-16-14(8-11)15(19)10-18(23-16)13-6-4-12(21-2)9-17(13)22-3/h4-10H,1-3H3
InChI 密鑰
WUWFDVDASNSUKP-UHFFFAOYSA-N
生化/生理作用
6, 2′, 4′-trimethoxyflavone is a selective aryl hydrocarbon receptor (AHR) antagonist with no partial agonist activity.
6, 2′, 4′-trimethoxyflavone is a selective aryl hydrocarbon receptor (AHR) antagonist with no partial agonist activity. The role of the transcription factor aryl hydrocarbon receptor (AHR) in biology is still under evaluation and has expanded beyond that of a xenobiotic sensor and regulator of detoxification. Inhibition of AHR activity by antagonists could result in anti-inflammatory actions. 6, 2′, 4′-trimethoxyflavone (TMF) is a pure AHR antagonist. The compound compete with agonists, such as 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) and benzo[a]pyrene (B[a]P), thus effectively inhibiting AHRmediated transactivation of a heterologous reporter and endogenous targets e.g. CYP1A1. TMF also exhibits no species or promoter dependency with regard to AHR antagonism. Thus it represents an improved tool allowing for more precise dissection of AHR function.
The AHR along with immune functions, is also associated with endocrine processes and cancer development.
準備報告
6, 2′, 4′-trimethoxyflavone is soluble in DMSO at a concentration that is greater than 5 mg/ml.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Róisín O'Flaherty et al.
Journal of proteome research, 16(11), 4237-4243 (2017-09-28)
Here we report evidence that new aminoquinoline N-glycan fluorescent labels interfere with the release of core α(1-6) fucose from N-glycans by bovine kidney α-l-fucosidase (BKF). BKF is a commonly employed exoglycosidase for core α(1-6) fucose determination. Molecular simulations of the
Noël J Diepens et al.
Environmental science & technology, 52(15), 8510-8520 (2018-06-22)
We present a generic theoretical model (MICROWEB) that simulates the transfer of microplastics and hydrophobic organic chemicals (HOC) in food webs. We implemented the model for an Arctic case comprised of nine species including Atlantic cod and polar bear as
Antagonism of aryl hydrocarbon receptor signaling by 6, 2?, 4?-trimethoxyflavone
Murray IA, et al.
Journal of Pharmacology and Experimental Therapeutics, 332(1), 135-144 (2010)
Runxia Sun et al.
Environmental pollution (Barking, Essex : 1987), 222, 165-174 (2017-01-04)
Short chain chlorinated paraffins (SCCPs) are under review for inclusion into the Stockholm Convention on Persistent Organic Pollutants. However, limited information is available on their bioaccumulation and biomagnification in ecosystems, which is hindering evaluation of their ecological and health risks.
Joseph Shailender et al.
Drug development and industrial pharmacy, 44(7), 1109-1119 (2018-02-08)
Design chitosan based nanoparticles for tenofovir disoproxil fumarate (TDF) with the purpose of enhancing its oral absorption. TDF is a prodrug that has limited intestinal absorption because of its susceptibility to gut wall esterases. Hence, design of chitosan based polymeric
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门