推荐产品
ligand
thalidomide
化驗
>98%
形狀
solid
光學活性
[α]23/D −62.6°, c = 2 in DMF(lit.)
反應適用性
reagent type: ligand
技術
cell culture | embryo: suitable
顏色
white
溶解度
DMSO: soluble
H2O: insoluble
ethanol: insoluble
起源
Celgene
SMILES 字串
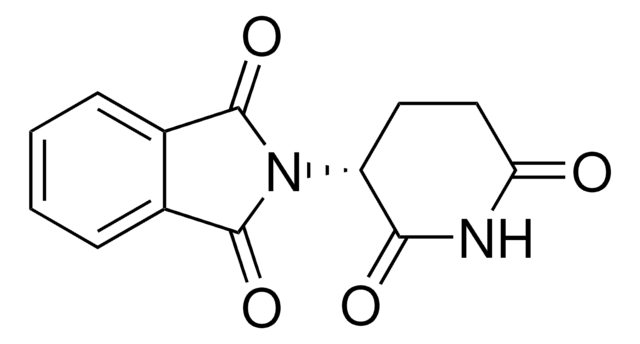
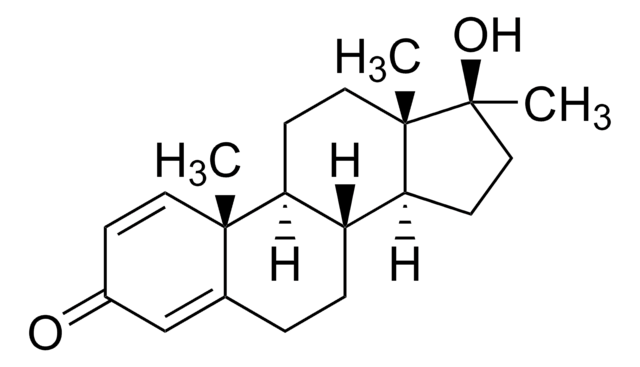
O=C1CC[C@H](N2C(=O)c3ccccc3C2=O)C(=O)N1
InChI
1S/C13H10N2O4/c16-10-6-5-9(11(17)14-10)15-12(18)7-3-1-2-4-8(7)13(15)19/h1-4,9H,5-6H2,(H,14,16,17)/t9-/m0/s1
InChI 密鑰
UEJJHQNACJXSKW-VIFPVBQESA-N
基因資訊
human ... LITAF(9516) , TNF(7124)
mouse ... Nos2(18126)
rat ... Nos1(24598)
一般說明
Thalidomide is a stereoisomer, which exists in two enantiomeric states. The S enantiomer is teratogenic. Thalidomide consists of two linked rings, a phthalimide and glutarimide ring.
應用
Thalidomide has been used to study its teratogenic effects in chicken embryos and human embryonic cells. This study reported that thalidomide causes limb defects by stabilizing PTEN, inhibiting the expression of Akt and activating caspase-dependent apoptosis. Thalidomide has also been used for studying glutathione mediated teratogenic resistance in mouse embryos.
生化/生理作用
(-)-Thalidomide selectively inhibits the biosynthesis of tumor necrosis factor α (TNF-α), which is essential for inflammatory response. It is an anti-emetic drug and is used to treat morning sickness in pregnant women. Thalidomide is also used to treat leprosy, multiple myeloma, Crohn′s disease and human immunodeficiency virus (HIV) infection. Thalidomide also inhibits angiogenesis. It is associated with several diseases such as, peripheral neuropathy, facial palsies, Duane syndrome and autism.
特點和優勢
This compound was developed by Celgene. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
準備報告
(-)-Thalidomide is soluble in DMSO, but is insoluble in water and ethanol.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Thalidomide-induced teratogenesis: History and mechanisms
Vargesson N
Birth Defects Research Part C: Embryo Today - Reviews, 105(2) (2015)
Thalidomide: the tragedy of birth defects and the effective treatment of disease
Kim JH and Scialli AR
Toxicological Sciences, 122(1) (2011)
Guo-Liang Duan et al.
International journal of biological macromolecules, 137, 1112-1120 (2019-07-05)
Six polysaccharides (SF-FB11, SF-HW21, SF-CA31, SF-HA41, SF-FF51, and SF-FR61) of similar molecular weights (MW) (30-50 kDa) were extracted from the fermentation liquor, mycelia, and basidiomata of Sparassis latifolia by different methods. Structural analyses of these purified polysaccharides indicated that they were
John H Sampson et al.
Molecular cancer therapeutics, 8(10), 2773-2779 (2009-10-15)
Conventional therapies for glioblastoma multiforme (GBM) fail to target tumor cells exclusively, such that their efficacy is ultimately limited by nonspecific toxicity. Immunologic targeting of tumor-specific gene mutations, however, may allow more precise eradication of neoplastic cells. The epidermal growth
Jürgen Knobloch et al.
Molecular pharmaceutics, 5(6), 1138-1144 (2009-05-13)
Thalidomide as an effective treatment for multiple myeloma and leprosy has also caused birth defects in thousands of children five decades ago particularly in Europe. Thus its use in humans remains limited. The rapid and fatal approval of thalidomide at
Chromatograms
application for HPLCapplication for HPLC我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门