推荐产品
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to beige
溶解度
H2O: 10 mg/mL (clear solution)
運輸包裝
wet ice
儲存溫度
−20°C
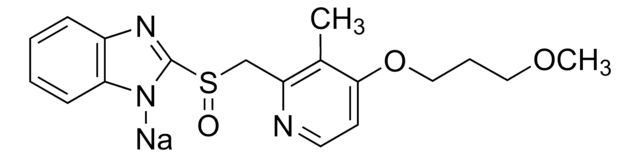
SMILES 字串
[Na+].COCCCOc1ccnc(CS(=O)c2nc3ccccc3[n-]2)c1C
InChI
1S/C18H20N3O3S.Na/c1-13-16(19-9-8-17(13)24-11-5-10-23-2)12-25(22)18-20-14-6-3-4-7-15(14)21-18;/h3-4,6-9H,5,10-12H2,1-2H3;/q-1;+1
InChI 密鑰
KRCQSTCYZUOBHN-UHFFFAOYSA-N
基因資訊
human ... ATP4A(495) , ATP4B(496)
正在寻找类似产品? 访问 产品对比指南
應用
Rabeprazole sodium has been used as a proton pump inhibitor (PPI) to study its effects on spontaneous uterine contractile activity in pregnant women.
生化/生理作用
Rabeprazole sodium is gastric proton pump inhibitor. It suppresses the production of acid in the stomach by inhibiting the gastric H+/K+ATPase (hydrogen-potassium adenosine triphosphatase) at the secretory surface of the gastric parietal cell. Rabeprazole sodium has been used clinically to treat acid-reflux disorders (GERD), peptic ulcer disease, H. pylori eradication, and prevent gastroinetestinal bleeds associated with NSAID use.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 4
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Increased gap density predicts weakness of the epithelial barrier in vivo by confocal laser endomicroscopy in indomethacin-induced enteropathy.
Shi S, Wang H, Gao H, et al.
Digestive Diseases and Sciences, 59(7), 1398-1405 (2014)
Hyun Jeong Lee et al.
The Journal of infectious diseases, 208(7), 1123-1130 (2013-06-27)
Clarithromycin-resistant Helicobacter pylori is associated with point mutations in the 23S ribosomal RNA (rRNA) gene. A total of 1232 patients participated and were divided into 2 control groups and 1 case group. Patients in the APC control group, which consisted
Tarek A Ahmed et al.
Life sciences, 110(1), 35-43 (2014-07-06)
First; to develop rabeprazole (RP)-alginate core coated chitosan nanoparticles (NP) utilizing water in oil (W/O) nanoemulsion technique. Second; formulation of transdermal patches loaded RP-NP that avoid drug peroral acid sensitivity and first pass effect. The influence of six factors on
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门