所有图片(1)
About This Item
经验公式(希尔记法):
C15H11BrNNaO3
CAS号:
分子量:
356.15
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
化驗
≥98% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
faintly yellow to dark yellow
溶解度
H2O: ≥5 mg/mL
儲存溫度
2-8°C
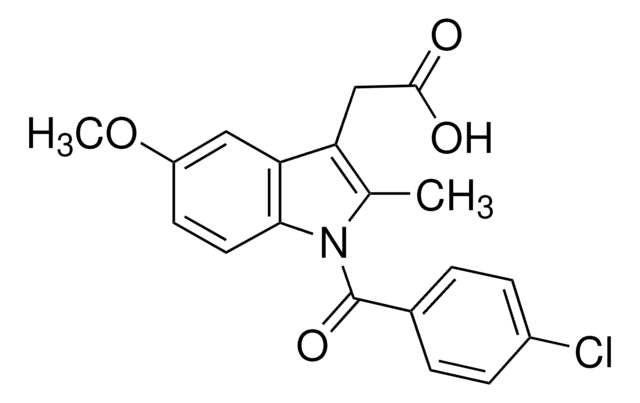
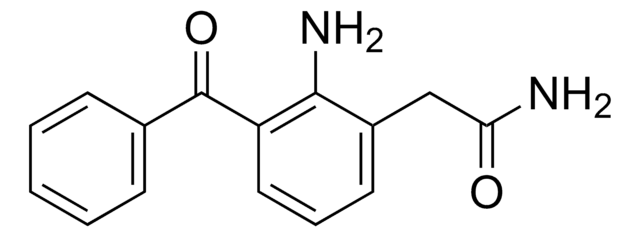
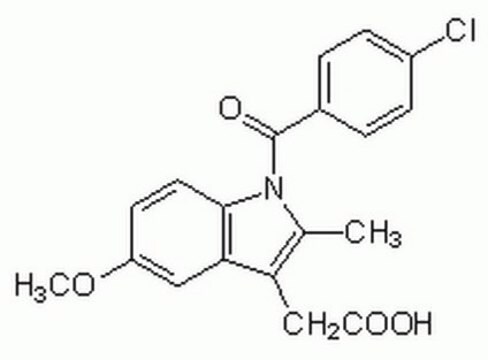
SMILES 字串
[Na+].Nc1c(CC([O-])=O)cccc1C(=O)c2ccc(Br)cc2
InChI
1S/C15H12BrNO3.Na/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19;/h1-7H,8,17H2,(H,18,19);/q;+1/p-1
InChI 密鑰
HZFGMQJYAFHESD-UHFFFAOYSA-M
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
應用
溴芬酸钠可用于:
- 研究其与黑色素的结合能力
- 合成溴芬酸吲哚二酮标准物
- 分析其在猪结膜中的渗透性
生化/生理作用
溴芬酸展示解热和抑制前列腺素合成酶的特性。它对缓解术后白内障患者的眼痛和炎症有治疗作用。溴芬酸是对抗变应性结膜炎的有效药剂。它有望成为治疗急性肌肉疼痛、骨关节炎和类风湿性关节炎的良药。
溴芬酸是一种非甾体抗炎药(NSAID);COX1 和 COX2 抑制剂;眼科止痛药。
溴芬酸是一种非甾体类抗炎药(NSAID),可同时抑制 COX1 和 COX2。它用作眼科止痛药。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Christina Flaxel et al.
Retina (Philadelphia, Pa.), 32(3), 417-423 (2011-08-25)
To evaluate whether bromfenac eyedrops and ranibizumab intravitreal injections would provide added efficacy over ranibizumab alone. This was a single-site, multiinvestigator, prospective, open-label, interventional, Phase II study of patients with new or recurrent exudative/neovascular age-related macular degeneration. Thirty eyes were
Jeffrey S Heier et al.
Retina (Philadelphia, Pa.), 29(9), 1310-1313 (2009-11-26)
To assess vitreous concentrations of nonsteroidal antiinflammatory drugs (NSAIDs) and prostaglandin E(2) in patients treated with NSAIDs before vitrectomy. This was an investigator-masked, randomized, multicenter study. Patients received ketorolac 0.4% 4 times a day, bromfenac 0.09% 2 times a day
Frank A Bucci et al.
Advances in therapy, 28(12), 1089-1095 (2011-11-23)
We compared the prostaglandin E(2) (PGE(2)) inhibition of three topical nonsteroidal antiinflammatory drugs (NSAIDs): ketorolac 0.45%, bromfenac 0.09%, and nepafenac 0.1% at peak dosing levels in patients previously scheduled to undergo phacoemulsification. This was a single-center, double-masked observational study of
Frank A Bucci et al.
Current medical research and opinion, 27(12), 2235-2239 (2011-10-14)
To compare the peak to-aqueous penetration of three nonsteroidal anti-inflammatory drugs: ketorolac tromethamine 0.45%, bromfenac 0.09%, nepafenac 0.1%, and amfenac (the active metabolite of nepafenac) in patients undergoing phacoemulsification. A single center, double-masked study randomized 122 patients to receive one
Koichiro Mukai et al.
Journal of cataract and refractive surgery, 35(9), 1614-1618 (2009-08-18)
To evaluate the efficacy of ophthalmic nonsteroidal and steroidal antiinflammatory drugs in preventing anterior capsule contraction and secondary posterior capsule opacification (PCO) using an experimental cataract model. Department of Ophthalmology, Dokkyo Medical University, Tochigi, Japan. Eight-week-old albino rabbits weighing about
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门