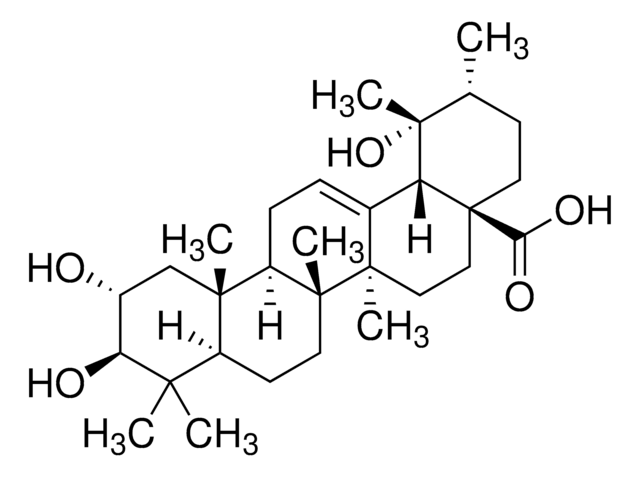
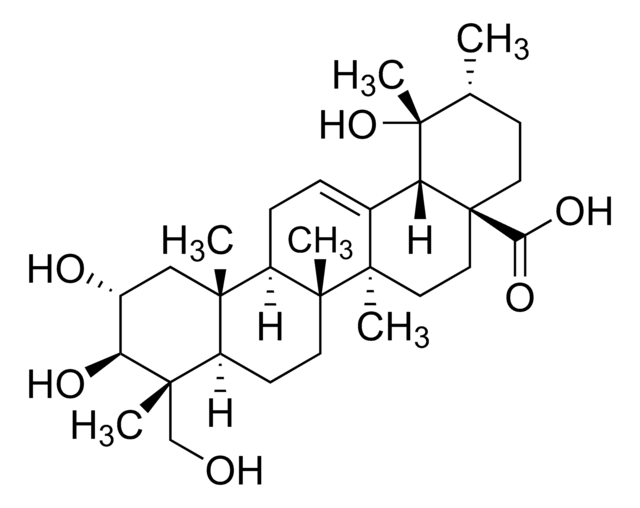
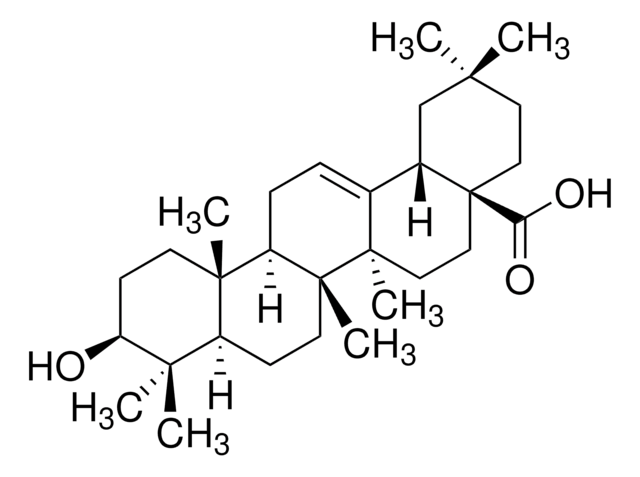
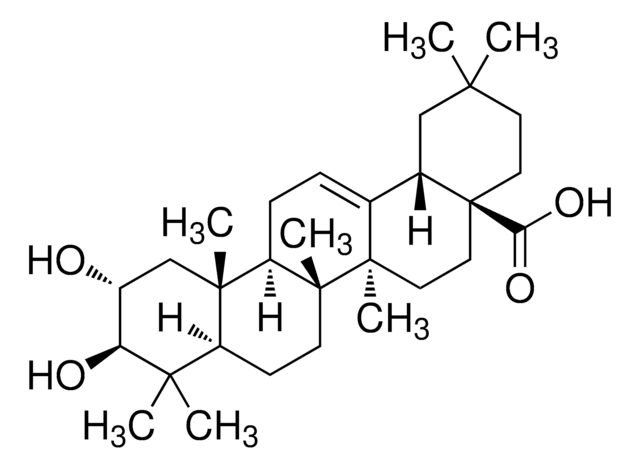
推荐产品
生物源
plant
化驗
≥90% (LC/MS-ELSD)
形狀
solid
分子量
472.7
溶解度
water: slightly soluble
應用
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
−20°C
InChI
1S/C30H48O4/c1-18-10-15-30(24(32)33)17-16-27(5)19(23(30)29(18,7)34)8-9-21-26(4)13-12-22(31)25(2,3)20(26)11-14-28(21,27)6/h8,18,20-23,31,34H,9-17H2,1-7H3,(H,32,33)/t18-,20+,21-,22+,23-,26+,27-,28-,29-,30+/m1/s1
InChI 密鑰
ZZTYPLSBNNGEIS-OPAXANQDSA-N
一般說明
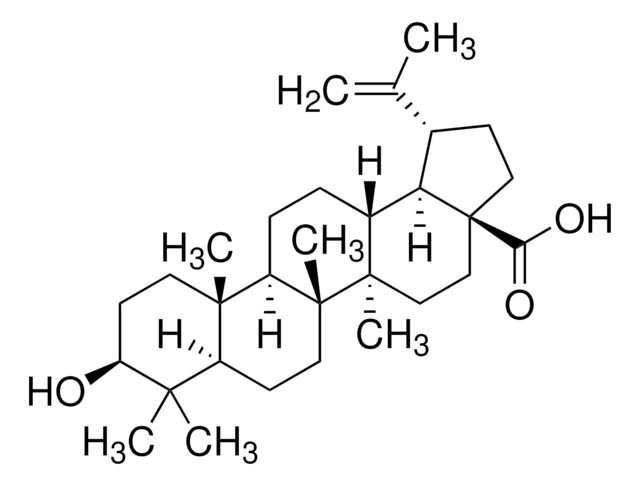
Pomolic acid, a pentacyclic triterpene, is a bioactive natural compound commonly derived from plants such as Euscaphis japonica, Centella asiatica, Picramnia sellowii, and Cecropia pachystachya. Current research suggests that this metabolite acts as an inhibitor and may exhibit diverse biological activities, including anticancer, anti-inflammatory, antiviral, apoptotic, and antihypertensive properties.
Natural product derived from plant source.
應用
Pomolic acid is a natural product derived from plant source that finds application in compound screening libraries, metabolomics, phytochemical, and pharmaceutical research.
生化/生理作用
Pomolic acid is highly effective in inhibiting cell growth and inducing apoptosis.
特點和優勢
- Suitable for Biochemical and Biomedical research
- Versatile and adaptable for wide variety of laboratory and research applications
其他說明
For additional information on our range of Biochemicals, please complete this form.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Guillermo Schinella et al.
Planta medica, 74(3), 215-220 (2008-02-09)
The dichloromethane extract and pomolic acid ( 5) obtained from leaves of Cecropia pachystachya both reduced carrageenan-induced paw oedema in mice. Interestingly, while the triterpenoid inhibited the in vivo production of interleukin-1beta by 39 %, it had no effect on
Phuong Thien Thuong et al.
Biological & pharmaceutical bulletin, 29(4), 830-833 (2006-04-06)
A new ursane-type triterpenoid, weigelic acid (1), and seven known compounds, ursolic acid (2), ilekudinol A (3), corosolic acid (4), ilekudinol B (5), esculentic acid (6), pomolic acid (7), and asiatic acid (8) were isolated from the leaf and stem
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门