推荐产品
物種活性
human
包裝
kit of 96 wells (12 strips x 8 wells)
技術
ELISA: suitable
capture ELISA: suitable
輸入
sample type urine
sample type serum
sample type cell culture supernatant(s)
sample type plasma
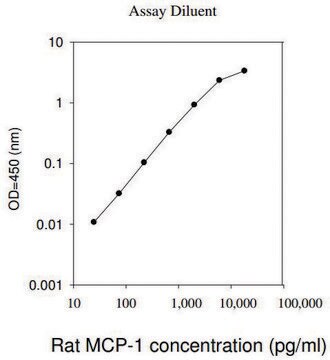
assay range
inter-assay cv: <12%
intra-assay cv: <10%
sensitivity: 5 pg/mL
standard curve range: 10.2-2500 pg/mL
檢測方法
colorimetric
運輸包裝
wet ice
儲存溫度
−20°C
基因資訊
human ... CCL17(6361)
一般說明
The Human TARC (Thymus and Activation Regulated Chemokine) ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro enzyme-linked immunosorbent assay for the quantitative measurement of human TARC in serum, plasma, cell culture supernatants and urine.
免疫原
Recombinant Human TARC
應用
请参考Protocol了解详情。
生化/生理作用
C-C motif chemokine ligand 17 (CCL17), also known as thymus and activation-regulated chemokine (TARC), is produced by bronchial epithelial cells, endothelial cells and myeloid dendritic cells. This chemokine is a ligand for CC chemokine receptors 4 and 8. It is involved in recruiting type 2 T-helper cells (Th2) cells and in activating other antigen-presenting cells. CCL17 has been linked to atopic dermatitis and asthma.
其他說明
A sample Certificate of Analysis is available for this product.
Please type the word sample in the text box provided for lot number.
Please type the word sample in the text box provided for lot number.
试剂盒组分也可单独购买
产品编号
说明
化学品安全说明书
訊號詞
Warning
危險聲明
防範說明
危險分類
Met. Corr. 1
儲存類別代碼
8A - Combustible corrosive hazardous materials
Low CCL17 expression associates with unfavorable postoperative prognosis of patients with clear cell renal cell carcinoma
Ying Xiong
BMC Cancer (2017)
Targeting CD1c-expressing classical dendritic cells to prevent thymus and activation-regulated chemokine (TARC)-mediated T-cell chemotaxis in rheumatoid arthritis
MR Hillen
Scandinavian Journal of Rheumatology. Supplement (2017)
Anna Guidetti et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 20(22), 5641-5651 (2014-09-23)
To evaluate safety and activity of perifosine and sorafenib combination therapy in patients with lymphoproliferative diseases. Patients with relapsed and refractory lymphoproliferative diseases received perifosine (50 mg twice daily) for 1 month. Patients achieving less than partial response (PR) after
Karen Knipping et al.
Acta paediatrica (Oslo, Norway : 1992), 103(7), 766-774 (2014-04-05)
Eosinophilic oesophagitis (EO) is an emerging worldwide disease, closely associated with male gender and allergic disorders. This study investigated the distribution of allergy markers in a cohort of children with EO. We analysed allergy markers in 91 children (62 males
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门